1821908-48-8
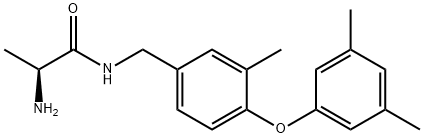 1821908-48-8 結構式
1821908-48-8 結構式
基本信息
CS-2529
SGC-2085
SGC 2085
Propanamide, 2-amino-N-[[4-(3,5-dimethylphenoxy)-3-methylphenyl]methyl]-, (2S)-
物理化學性質(zhì)
常見問題列表
IC50: 50 nM (CARM1)
SGC2085 which features a methyl at position R1 and a 3,5-dimethylphenoxy at R2 has an IC 50 of 50 nM for CARM1 and is over 100-fold selective for CARM1 over PRMT6. These results indicate that the presence of a substituent at R1 is essential for potent and selective inhibition of CARM1. With the exception of PRMT6 (IC 50 =5.2 μM), SGC2085 does not inhibit other PRMTs. Considering its small size (MW=312.4 Da), SGC2085 has an excellent selectivity profile, which can probably be further improved by exploiting differences in the binding sites of the two enzymes outside the arginine binding pocket. Compound SGC2085 also shows complete selectivity against a panel of 21 human protein methyltrans- ferases tested at three different concentrations (1,10, and 50 μM). To characterize the mechanism of action of SGC2085 in solution, IC 50 values are determined at various concentrations of SAM and peptide substrate. Increasing concentration of substrate peptide or cofactor does not affect IC 50 values, indicative of a noncompetitive mechanism of inhibition, which has been previously shown for other protein methyltransferase inhibitors binding at the substrate pocket. No cellular activity is observed for SGC2085 when tested up to 10 μM (48 h exposure in HEK293 cells), while methylation of BAF155 is abrogated by 10 μM of the dual CARM1/PRMT6 inhibitor MS049. We assume that the absence of cellular activity for SGC2085 is due to poor permeability.