| Identification | More | [Name]
Carbazole | [CAS]
86-74-8 | [Synonyms]
9-AZAFLUORENE
9H-CARBAZOLE
9H-DIBENZO(B,D)PYRROLE
AKOS BBS-00004359
CARBAZOLE
DIBENZO[B D]PYRROLE
DIBENZOPYRROLE
DIPHENYLENIMINE
DIPHENYLIMIDE
'LGC' (2409)
9-Dibenzo-[b,d]-pyrrole
Diphenylenedimine
Diphenyleneimide
Diphenyleneimine
Diphenylenimide
Skf 20091
USAF ek-600
usafek-600
Carbazoletechn
Melting point standard Carbazole | [EINECS(EC#)]
201-696-0 | [Molecular Formula]
C12H9N | [MDL Number]
MFCD00004960 | [Molecular Weight]
167.21 | [MOL File]
86-74-8.mol |
| Chemical Properties | Back Directory | [Appearance]
white crystals or light brown powder | [Melting point ]
243-246 °C (lit.) | [Boiling point ]
355 °C (lit.) | [bulk density]
620kg/m3 | [density ]
1.1 | [vapor pressure ]
400 mm Hg ( 323 °C)
| [refractive index ]
1.6192 (estimate) | [Fp ]
220 °C
| [storage temp. ]
2-8°C | [solubility ]
acetone: soluble50mg/mL | [form ]
Crystalline Powder, Flakes, or Chunks | [pka]
17.00±0.30(Predicted) | [color ]
Beige-yellow or beige-brownish | [Odor]
char. odor | [Stability:]
Stable. Combustible. Incompatible with strong oxidizing agents, nitrogen oxides, potassium hydroxide. | [Water Solubility ]
<0.1 g/100 mL at 19 ºC | [Detection Methods]
GC,NMR | [Merck ]
14,1790 | [BRN ]
3956 | [Dielectric constant]
1.3(Ambient) | [InChIKey]
UJOBWOGCFQCDNV-UHFFFAOYSA-N | [LogP]
3.84 at 22℃ and pH7 | [CAS DataBase Reference]
86-74-8(CAS DataBase Reference) | [IARC]
2B (Vol. 32, Sup 7, 71, 103) 2013 | [NIST Chemistry Reference]
Carbazole(86-74-8) | [Storage Precautions]
Store under nitrogen | [EPA Substance Registry System]
86-74-8(EPA Substance) |
| Safety Data | Back Directory | [Hazard Codes ]
Xn,N,T | [Risk Statements ]
R22:Harmful if swallowed.
R36/37/38:Irritating to eyes, respiratory system and skin .
R40:Limited evidence of a carcinogenic effect.
R50/53:Very Toxic to aquatic organisms, may cause long-term adverse effects in the aquatic environment .
R63:Possible risk of harm to the unborn child.
R43:May cause sensitization by skin contact.
R23/24/25:Toxic by inhalation, in contact with skin and if swallowed .
R45:May cause cancer. | [Safety Statements ]
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice .
S36:Wear suitable protective clothing .
S60:This material and/or its container must be disposed of as hazardous waste .
S61:Avoid release to the environment. Refer to special instructions safety data sheet .
S36/37:Wear suitable protective clothing and gloves .
S24/25:Avoid contact with skin and eyes .
S23:Do not breathe gas/fumes/vapor/spray (appropriate wording to be specified by the manufacturer) .
S53:Avoid exposure-obtain special instruction before use .
S45:In case of accident or if you feel unwell, seek medical advice immediately (show label where possible) . | [RIDADR ]
UN 2811 6.1/PG 3
| [WGK Germany ]
2
| [RTECS ]
FE3150000
| [F ]
10 | [Hazard Note ]
Harmful | [TSCA ]
Yes | [HazardClass ]
9 | [PackingGroup ]
III | [HS Code ]
29339990 | [Safety Profile]
intraperitoneal route. A flammable liquid. | [Hazardous Substances Data]
86-74-8(Hazardous Substances Data) | [Toxicity]
LD50 orally in rats: >5 g/kg (Eagle, Carlson) |
| Hazard Information | Back Directory | [General Description]
White crystals, plates, leaflets or light tan powder. Sublimes readily. Exhibits strong fluorescence and long phosphorescence on exposure to ultraviolet light. | [Reactivity Profile]
CARBAZOLE(86-74-8) is an extremely weak base. CARBAZOLE(86-74-8) is incompatible with strong oxidizing agents. CARBAZOLE(86-74-8) reacts with nitrogen oxides. Potassium hydroxide fusion yields a salt. | [Air & Water Reactions]
Insoluble in water. | [Hazard]
Possible carcinogen. | [Fire Hazard]
Flash point data for this chemical are not available; however, CARBAZOLE is probably combustible. | [Chemical Properties]
white crystals or light brown powder | [Uses]
Important dye intermediate. Used in making photographic plates sensitive to ultraviolet light. Reagent for lignin, carbohydrates, and formaldehyde. | [Application]
Carbazole can be used to produce dyes, pigments, photoconductors, photosensitive materials, special inks, etc. The pigment produced with it is permanent purple RL, which is widely used in the coloring of automobile topcoat and high temperature resistant plastics, and has the advantages of high temperature resistance and ultraviolet light resistance. The dyestuffs sulphur vat blue RNX and Haichang blue produced with it have excellent fastness indicators, especially the fastness to chlorine bleaching. The blue varieties include carbazole IDM, carbazole LR, carbazole LB, and carbazole L3B. , the black varieties have carbazole black D. It also produces carbazole bisoxazine violet, a blue-violet pigment used in coatings, printing inks, carbon paper, and more. Carbazole is used in the production of sulfide reduced blue RNX, direct lightfast blue FFRL, FFGL, etc. It can also make leather, N-vinylcarbazole plastics, pesticides and insecticides tetranitrocarbazole, chlorinated carbazole, and UV-sensitive photographic dry films. In addition, carbazole has been increasingly used in the development of emerging optoelectronic new materials. The use of carbazole can prepare organic nonlinear optics (NLO) materials, organic electroluminescence (OEL) materials, photorefractive materials, containing Bifunctional system of carbazole chromophore, carbazole-containing photorefractive small molecular glass, etc. | [Definition]
A
white crystalline compound used in the
manufacture of dyestuffs. | [Definition]
carbazole: A white crystalline compoundfound with anthracene,C12H9N; m.p. 238°C; b.p. 335°C. It isused in the manufacture of dyestuffs. | [Synthesis Reference(s)]
Tetrahedron Letters, 14, p. 761, 1973 DOI: 10.1016/S0040-4039(01)95705-3 | [Biological Activity]
Carbazole mainly interacts with DNA and damages it. These events suppress the synthesis of new DNA or RNA. Carbazole derivatives are involved in antimicrobialantitumorantiepilepticantihistaminicantioxidantanti-inflammatoryantidiarrhoealanalgesicneuroprotectiveand pancreatic lipase suppression activities. | [Solubility in organics]
Very weak alkaline. Slightly soluble in ethanol, ether and benzene, soluble in quinoline, pyridine and acetone, slightly soluble in cold benzene, glacial acetic acid, chloroform, carbon disulfide and gasoline, soluble in concentrated sulfuric acid without decomposition, slightly soluble in petroleum ether, chlorinated hydrocarbons and acetic acid. | [Purification Methods]
Dissolve carbazole (60g) in conc H2SO4 (300mL), extract with three 200mL portions of *benzene, then stir this into 1600mL of an ice-water mixture. The precipitate is filtered off, washed with a little water, dried, recrystallised from *benzene and then from pyridine/*benzene [Feldman et al. J Am Chem Soc 73 4341 1951]. It has also been recrystallised from EtOH or toluene, sublimed in vacuum, zone-refined, and purified by TLC. [UV: Armarego in Physical Methods in Heterocyclic Chemistry (Ed Katritzky, Academic Press) Vol III 158 1971, Beilstein 20/8 V 9.] | [Toxics Screening Level]
The Initial Risk Screening Level (IRSL) for carbazole is 0.4 μg/m3 based on an annual averaging time. |
| Questions And Answer | Back Directory | [Introduction]
Carbazole and its derivatives are a class of important nitrogen-containing heterocyclic compounds possessing various unique properties and biological activity.
Carbazole is a colorless small scale crystal, insoluble in water and inorganic acid, slightly soluble in ethanol, ether, acetone, benzene (in high temperature), soluble in chloroform, glacial acetic acid, carbon disulfide, pyridine and furfural, etc. It has intense fluorescence and prolonged phosphorescence under ultraviolet light.
| [Extraction]
Sulfuric acid method
Dissolve crude anthracene in chlorobenzene or other solvents to remove soluble phenanthrenes, quinones, etc. Insoluble anthracene and carbazoles are then reacted with concentrated sulfuric acid, and then carbazole sulfate is generated departing from the anthracene. The carbazole sulfate is hydrolyzed, filtered and dried to obtain carbazole.
Solvent-rectification method
Dissolve crude anthracene with heavy benzene, and remove soluble phenanthrene, quinone and other substances. Insoluble anthracene and carbazole are rectified in a rectification tower to obtain a mixture containing 85 to 90% of carbazole with a yield of 65%.
Pyridine solvent method
Dissolve crude anthracene with heavy benzene, and remove soluble phenanthrene, quinone and other substances. Then pyridine is used as a solvent to filter off insoluble anthracene at 90°C, and the filtrate is then crystallized to obtain crude carbazole. The crude carbazole can be treated with chlorobenzene or other solvents to obtain carbazole with yield of 97 to 99%.
| [Production]
a. Graebe-Ullmann:
Diazotization from amino diphenylamine, and nitrogen gas heated off to generate carbazole. Reported by Graebe and Ullmann in 1896.
b. Bucherer:
Co-thermal reaction of aryl hydrazine and naphthol in the presence of sodium bisulfite. Reported by Bucherer in 1904.
c. Borsche Drechsel:
Hydrazone is synthesized by condensation of Benzoquinone and cyclohexanone, and the latter cyclized to tetrahydrocarbazole under acidic conditions and then catalyzed by dehydrogenation to generate carbazole. Reported by Drechsel and Borsche in 1868 and 1904 respectively.
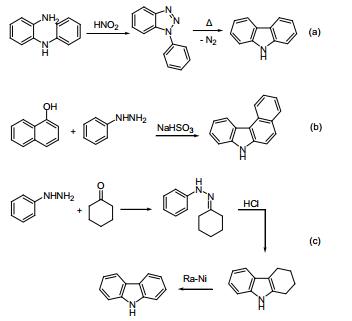
Thanks to the development of the catalytic dehydrogenation method, the Borsche method has become a carbazole synthetic route with simple operation, mild conditions, low cost and high yield, and has a high industrial production value.
Because of its unique structure and physicochemical properties, carbazole has aroused great interest among researchers. In recent years, novel monoand poly-substituted carbazole derivatives have been found to have good anti-tumor and anti-convulsant activities, showing broad application prospects. The demand for carbazole and its derivatives has been increasing. In the last decade of the 20th century, a large number of novel precious metals and transition metal catalysts have been used in the synthesis of carbazoles and their derivatives, which greatly enriched the synthetic methods and lay the foundation of synthesizing more complex carbazole derivatives. Nowadays, not only carbazole, but tens of thousands of carbazole derivatives are synthesized by new methods, and are widely used in the pharmaceutical and dye industries. |
|
|