| Identification | Back Directory | [Name]
UROTENSIN I | [CAS]
83930-33-0 | [Synonyms]
UROTENSIN I
Urotensin IUrotens
Catostomus urotensin I
urotensin i teleost fish
Urotensin I, ≥95% (HPLC)
Urotensin I (Catostomuscommersoni) (9CI)
NDDPPISIDLTFHLLRNMIEMARIENEREQAGLNRKYLDEV-NH2
ASN-ASP-ASP-PRO-PRO-ILE-SER-ILE-ASP-LEU-THR-PHE-HIS-LEU-LEU-ARG-ASN-MET-ILE-GLU-MET-ALA-ARG-ILE-GLU-ASN-GLU-ARG-GLU-GLN-ALA-GLY-LEU-ASN-ARG-LYS-TYR-LEU-ASP-GLU-VAL-NH2
H-ASN-ASP-ASP-PRO-PRO-ILE-SER-ILE-ASP-LEU-THR-PHE-HIS-LEU-LEU-ARG-ASN-MET-ILE-GLU-MET-ALA-ARG-ILE-GLU-ASN-GLU-ARG-GLU-GLN-ALA-GLY-LEU-ASN-ARG-LYS-TYR-LEU-ASP-GLU-VAL-NH2 | [Molecular Formula]
C210H340N62O67S2 | [MDL Number]
MFCD00082128 | [Molecular Weight]
4869.45 |
| Questions And Answer | Back Directory | [Discovery]
The presence of UI was first reported in 1982 after it was obtained from the urophysis of the white sucker Catostomus commersoni. Subsequently, UI has been found not only in teleosts but also in elasmobranchii (the dogfish shark Scyliorhinus canicula). | [Structure]
UI consists of 41 aa residues with an amidated C-terminus.Amino acid residues 4–19 arenecessary for adrenocorticotropic hormone (ACTH) secretion. The C-terminus is required for complete ACTH-secreting activity. The alignment of UI shows that UI forms two structurally separate groups, one including Pleuronectiformes (flounder), and the other including Cypriniformes (white sucker and goldfish) and Salmoniformes (salmonids). The sequence homology within the group is high (90%– 95%) while that between groups is low (59%–71%).
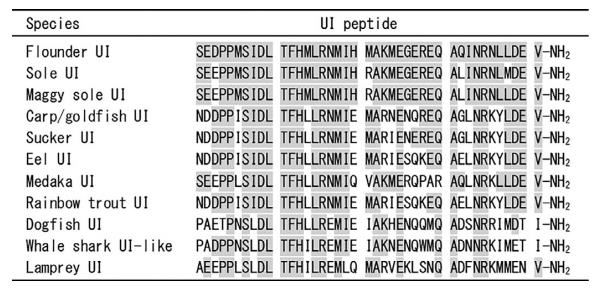 | [Properties]
Sucker UI: Mr 4870; pI 4.6. Freely soluble in water. Oxidation of methionine remarkably reduces UI’s blood-pressure decreasing activity. | [Biological functions]
UI was first isolated from the urophysis of the white sucker and has been shown to decrease blood pressure in the rat. UI modulates cortisol secretion either directly by acting on the steroidogenic cells of the interrenal tissue or indirectly via the hypothalamo-pituitary-adrenal (HPA) axis. The intraperitoneal injection of UI increases plasma cortisol levels in goldfish. UI stimulates ACTH release from superfused goldfish anterior pituitary cells; it also stimulates thyroid-stimulating hormone secretion from the pituitary cells of the coho salmon in vitro. UI is involved in the adaptability of fish regarding, for example, ion and fluid equilibrium, cardiovascular activity, and glucocorticoid release. In addition to the biological function on the HPA axis, a central injection of carp/goldfish UI suppresses food intake in a dosedependent manner in goldfish. The administration of UI also inhibits food intake in neonatal chicks. | [Synthesis and release]
In goldfish, the UI cDNA (769 bp) encodes a 146-aa precursor that consists of a signal peptide, a cryptic region, and a 41-aa mature peptide at the C-terminal. The flounder UI gene consists of two exons and one intron, and the whole precursor-coding region exists in the second exon. UI is produced from the prohormone by endopeptidase. Basic amino acid residues (Lys-Arg) exist in the N-terminal of UI for processing. The cleavage site in the C-terminal region is located at Gly-Lys; the Lys residue is cleaved by an exopeptidase and the Gly residue is then used as an amidation donor. Distribution of mRNA UI mRNA is mainly detected in the urophysis rather than in the brain in carp and rainbow trout, suggesting that UI plays its major role in peripheral tissues and has a relatively small role in the brain. In the goldfish brain, UI mRNA is mainly expressed in the telencephalon/preoptic area, and also in the optic tectum/thalamus, hypothalamus, cerebellum, and medulla oblongata, but not in the olfactory bulb and pituitary. In the white sucker brain, UI mRNA is detected in the preoptic region and the nucleus of the lateral tuberis. UI mRNA is elevated in the nucleus of the lateral tuberis by removal of the urophysis in the white sucker. |
| Hazard Information | Back Directory | [Description]
A peptide hormone first isolated from the urophysis of the white sucker, urotensin-I, has been shown to decrease blood pressure in the rat. | [Uses]
Urotensin I (Catostomus urotensin I), a CRF-like neuropeptide, acts as an agonist of CRF receptor with pEC50s of 11.46, 9.36 and 9.85 for human CRF1, human CRF2 and rat CRF2α receptors in CHO cells, and Kis of 0.4, 1.8, and 5.7 nM for hCRF1, rCRF2α and mCRF2β receptors, respectively[1][2]. | [in vivo]
Intraperitoneal injections of urotensin I, a CRF-like neuropeptide isolated from the caudal neurosecretory system of the teleost Catostomus commersoni, ovine CRF and sauvagine all produced significant increases in circulating levels of plasma cortisol in goldfish in which endogenous ACTH secretion was suppressed with betamethasone[3]. | [References]
[1] Smart D, et al. Characterisation using microphysiometry of CRF receptor pharmacology. Eur J Pharmacol. 1999 Aug 27;379(2-3):229-35. DOI:10.1016/s0014-2999(99)00506-3
[2] Reul JM, et al. Corticotropin-releasing factor receptors 1 and 2 in anxiety and depression. Curr Opin Pharmacol. 2002 Feb;2(1):23-33. DOI:10.1016/s1471-4892(01)00117-5
[3] Fryer J, et al. Urotensin I, a CRF-like neuropeptide, stimulates acth release from the teleost pituitary. Endocrinology. 1983;113(6):2308-2310. DOI:10.1210/endo-113-6-2308
[4] Gerritsen ME, et al. Urotensin I effects on intracellular content of cyclic AMP in the rat tail artery. Eur J Pharmacol. 1979;60(2-3):211-220. DOI:10.1016/0014-2999(79)90220-6 |
|
|