| Identification | Back Directory | [Name]
Gibberellic acid | [CAS]
77-06-5 | [Synonyms]
GA3
GIB
UVEX
ryzup
Maxon
Ralex
GibGro
GIBREL
GIBBEX
pgr-iv
Grocel
Gib-Sol
gibefol
Gibbrel
Regulex
RELEASE
Brellin
BERELEX
ACTIVOL
Pro-Gib
ArbostiM
PRO-GIBB
CEKU-GIB
KRI-GIBB
Gib-Tabs
Gibrescol
Gibreskol
Gibb-tabs
GA4 AND 7
activolga
NSC 14190
NSC 19450
Grocel GA3
Release LC
GIBERELLIN
NCI-C55823
GIBB ACID 3
,4a-lactone
RYZUPSTRONG
GIBBERELLIN
GA3/CH78217
Gibberellic
pro-gibbplus
Gibberellins
Gibberellin 1
Pro-Gibb Plus
GIBBERELLIN A
GIBBERELLIN X
Pro-Gibb(ICI)
GIBBERELLIN A3
TU 64-3-103-75
Gibberellic a
GIBBERLIC ACID
Gibberellin GA3
GIBBERELIC ACID
GIBERILLIC ACID
GIBBERELLIC ACID
Gibberillic acid
G.bberellic acid
Gibberellins A4A7
acidegibberellique
Giberellic acid Mix
Gibberellic Acid A3
Gibberellic acid,99%
gibberellins Berelex
Gibberelin (alpha X)
GIBBERELLIC ACID GA3
Gibberellic Acid 90%
GA3, Gibberellin A3
Gibberellic acid E.C.
Gibberellins A3 powder
gibberellina4mixt.with
GA3, Gibberellic acid
Gibberellic Acid(GA0)
Gibberellic Acid, powder
Gibberellic Acid, crystal
Gibberellic Acid (200 mg)
GIBBERELLIC ACID GA3 90+%
Gibberellic Acid (1.06020)
(+)-GIBBERELLIC ACID, 90+%
2beta-Hydroxygibberellin 1
Gibberellin,Gibberellicacid
GibberellicAcid(Ga3)90%Min.
Gibberellic acid, tech. 90%
dicarboxylicacid1,4a-lactone
GIBBERELLIC ACID, 100MG, NEAT
Gibberellic acid >=95% (HPLC)
GIBBERELLIC ACID FOR SYNTHESIS
GIBBERELLICACID,POWDER,90%PURE
Peroxide, bis(1-oxotetradecyl)-
Gibberellic Acid (200 mg) (FCC)
GIBBERELLICACID,A3(GA3)90%(BULK
Tetradecanoyl tetradecaneperoxoate
GIBBERELLICACID,A4+7(GA4+7)90%(BULK
gibberellina4mixt.withgibberellina7
,10beta-dicarboxylicacid1,4a-lactone
GIBBERELLIC ACID HORTICULTURAL GRADE
Gibberellic acid,GA3, Gibberellin A3
Gibberellic Acid, 90 Percent+, Powder
GIBBERELLIC ACID GA3 PESTANAL, 250 MG
GibberellicAcid-(PlantGrowthStimulator)
-3-ene-1,10-dicarboxylicacid1,4a-lactone
1alpha,10beta-dicarboxylicacid1-4alactone
gibberellina4mixt.withgibberellina7chemical
GIBBERELLIC ACID PLANT CELL*CULTURE TEST ED
(1alpha,2beta,4aalpha,4bbeta,10beta)-a-lacton
GIBBERELLIC ACID(GIBBERELLIN-A3) CRYST. PRACT.
Gibberellic acid, in dibasic ammonium phosphate
ro-4a,7-methano-9b,3-propeno[1,2-b]furan-4-carboxylicacid
ro-4a,7-methano-9b,3-propenoazuleno[1,2-b]furan-4-carboxylicacid
-ethano-3,8b-prop-1-enoperhydroindeno[1,2-b]furan-4-carboxylicacid
ethano-3,8b-prop-l-enoperhydroindeno-(1,2-b)furan-4-carboxylicacid
10-dicarboxylicacid,2,4a,7-trihydroxy-1-methyl-8-methylene-gibb-3-ene-1
7-trihydroxy-1-methyl-8-methylenegibb-3-ene-1,10-dicarboxylicacid1,4a-4a
4aalpha,7-trihydroxy-1beta-methyl-8-methylene-4aalpha,4bbeta-gibb-3-ene-2bet
4alpha,7-trihydroxy-1-methylene-4aalpha,4bbeta-gibb-3-ene-1alpha,10beta-2bet
3as,4s,4as,6s,8ar,8br,11s)-6,11-dihydroxy-3-methyl-12-methylene-2-oxo-4a,6-3
gibb-3-ene-1,10-dicarboxylicacid,2,4a,7-trihydroxy-1-methyl-8-methylene-,1,4
2,4a,7-trihydroxy-1-methyl-8-methylenegibb-3-ene-1,10-carboxylicacid1-4-lacto
2,4a,7-trihydroxy-1-methyl-8-methylenegibb-3-ene-1,10-dicarboxylicacid1,4a-la
(3s,3ar,4s,4as,7s,9ar,9br,12s)-7,12-dihydroxy-3-methyl-6-methylene-2-oxoperhyd
(3s,3as,4s,4as,6s,8as,8bs,11s)-6,11-dihydroxy-3-methyl-12-methylene-2-oxo-4a,6
(3s,3as,4s,4as,7s,9ar,9br,12s)-7,12-dihydroxy-3-methyl-6-methylene-2-oxoperhyd
Trihydroxy-1-methyl-8-methylenegibb-3-ene-1,10-dicarboxylic acid, 1,4a-lactone
(1alpha,2beta,4aalpha,4bbeta,10beta)-2,4a,7-trihydroxy-1-methyl-8-methylenegibb
2beta,4alpha,7-trihydroxy-1-methyl-8-methylene-4aalpha,4bbeta-gibb-3-ene-1alpha
ent-3a,10,13-Trihydroxy-20-norgibberella-1,16-diene-7,19-dioicacid19,10-lactone
2,4a,7-Trihydroxy-1-methyl-8-methylenegibb-3-ene-1,10-carboxylic acid 1-4-lactone
2,4a,7-Trihydroxy-1-methyl-8-methylenegibb-3-ene-1,10-dicarboxylic acid 1,4a-lacto
2,4A,7-TRIHYDROXY-1-METHYL-8-METHYLENEGIBB-3-ENE-1,10-DICARBOXYLIC ACID 1,4A-LACTONE
2�,4α,7-thihydroxy-1-methyl-8-methjylenegibb-3-ene-1,10-dicarboxylic acid-1,4α-lactone
4a,1-(Epoxymethano)-7,9a-methanobenz[a]azulene, gibb-3-ene-1,10-dicarboxylic acid deriv.
(3S,3aS ,4S,4aS,7S,9aR,12S)-7,12-dihydroxy-3-methyl-6-methyllene-2-oxoperhydro-4a,7-methano-9b
(1α,2β,4aα,4bβ,10β)-2,4a,7-Trihydroxy-1-Methyl-8-Methylenegibb-3-ene-1,10-dicarboxylic Acid 1,4a-Lactone
10-dicarboxylicacid,2,4.alpha.,7-trihydroxy-1-methyl-8-methylene-,1,4a-lactone,(1.alpha.,2.beta.Gibb-3-ene-1
Gibb-3-ene-1,10-dicarboxylic acid, 2,4a,7-trihydroxy-1-methyl-8-methylene-, 1,4a-lactone, (1α,2β,4aα,4bβ,10β)-
10-dicarboxylic acid, 2,4.alpha.,7-trihydroxy-1-methyl-8-methylene-, 1,4a-lactone, (1.alpha.,2.beta.Gibb-3-ene-1
(1alpha,2beta,4aalpha,4bbeta,10beta)-2,4a,7-Trihydroxy-1-methyl-8-methylgibb-3-ene-1,10-dicarboxylic acid 1,4a-lactone
(1alpha,2beta,4aalpha,4bbeta,10beta)-2,4a, 7-Trihydroxy-1-methyl-8-methylenegibb-3-ene-1,10-dicarboxylic acid 1,4a-lactone
Gibb-3-ene-1,10-dicarboxylic acid, 2,4a,7-trihydroxy-1-methyl-8-methylene-, 1,4a-lactone, (1alpha,2beta,4aalpha,4bbeta,10beta)-
Gibb-3-ene-1,10-dicarboxylic acid, 2,4a,7-trihydroxy-1-methyl-8-methylene-, 1,4a-lactone, (1.alpha.,2.beta.,4a.alpha.,4b.beta.,10.beta.)-
(3S,3aR,4S,4aR,7S,9aR,9bR,12S)-7,12-dihydroxy-3-methyl-6-methylene-2-oxoperhydro-4a,7-methano-9b,13-propenoazuleno[1,2-b]furan-4-carboxylic acid
(3S,3aS,4S,4aS,6S,8aR,8bR,11S)-6,11-dihydroxy-3-methyl-12-methylene-2-oxo-4a,6-ethano-3,8b-prop-1-enoperhydroindeno[1,2-b]furan-4-carboxylic acid | [EINECS(EC#)]
201-001-0 | [Molecular Formula]
C19H22O6 | [MDL Number]
MFCD00079329 | [MOL File]
77-06-5.mol | [Molecular Weight]
346.37 |
| Chemical Properties | Back Directory | [Appearance]
white powder | [Melting point ]
227 °C
| [alpha ]
82.5 º (c=10, ethanol) | [Boiling point ]
401.12°C (rough estimate) | [density ]
1.34 g/cm3 (20℃) | [refractive index ]
81 ° (C=2, MeOH) | [storage temp. ]
0-6°C | [solubility ]
5g/l | [form ]
Powder | [pka]
4.0(at 25℃) | [color ]
White to pale yellow | [Stability:]
Stable. Combustible. Incompatible with acids, strong oxidizing agents. | [optical activity]
[α]20/D +80±3°, c = 1% in methanol | [Water Solubility ]
5 g/L (20 º C) | [Merck ]
14,4419 | [BRN ]
54346 | [InChIKey]
IXORZMNAPKEEDV-OBDJNFEBSA-N | [LogP]
0.240 | [CAS DataBase Reference]
77-06-5 | [NIST Chemistry Reference]
Gibberellic acid(77-06-5) | [EPA Substance Registry System]
Gibb-3-ene-1,10- dicarboxylic acid, 2,4a,7-trihydroxy-1-methyl- 8-methylene-, 1,4a-lactone, (1.alpha.,2.beta.,4a.alpha., 4b.beta.,10.beta.)-(77-06-5) |
| Hazard Information | Back Directory | [Chemical Properties]
white powder | [Uses]
Gibberellic Acid is a a pentacyclic diterpene acid. Gibberellic Acid is a hormone that is found in plants which promotes the growth and elongation of cells. Gibberellic acid acts as a plant growth reg
ulator on account of its physiological and morphological effects in extremely low concentrations. Generally Gibberellic acid affects only the plant parts above the soil surface. | [Definition]
ChEBI: A C19-gibberellin that is a pentacyclic diterpenoid responsible for promoting growth and elongation of cells in plants. | [General Description]
White powder. | [Air & Water Reactions]
Slightly water soluble . | [Reactivity Profile]
Gibberellic acid is readily degraded by acids. . | [Fire Hazard]
Flash point data for Gibberellic acid are not available. Gibberellic acid is probably combustible. | [Biosynthesis]
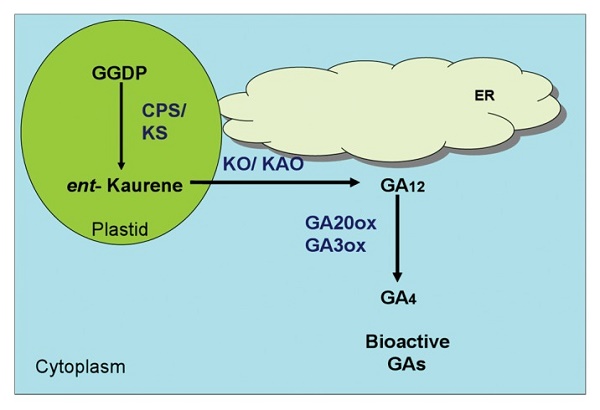
The presence of GA in actively growing tissues, i.e., shoot apices, young leaves, and flowers. Gibberellins (GAs; Gibberellic acids) being synthesized via the terpenoid pathway, require 3 enzymes viz., terpene synthase (TPSs), cytochrome P450 monooxygenase (P450s) and 2-oxoglutarate dependent dehydrogenase (2 ODDs), for the biosynthesis of bioactive GA from geranylgeranyl diphosphate in plants. Two terpene synthases, ent-copalyl diphosphate synthase (CPS) and ent-kaurene synthase (KS), located in plastids, are involved in the conversion of GGDP to tetracyclic hydrocarbon intermediate ent-kaurene. ent-Kaurene is then converted to GA12 by 2 P450s. First, ent-Kaurene oxidase (KO), present in the plastid's outer membrane, catalyzes the sequential oxidation of C-19 to produce ent-kaurenoic acid. Second, the kaurenoic acid oxidase (KAO) in the endoplasmic reticulum is converted to GA12. Bioactive GA4 is converted from GA12 through oxidations on C-20 and C-3 by GA 20-oxidase (GA20ox) and GA 3-oxidase (GA3ox)[3].
 | [Agricultural Uses]
Gibberellic acid is an organic acid used as a plant growth hormone. It is formed by the fungus Gibberellafujikuroi in aerated submerged culture. | [Agricultural Uses]
Gibberellin is a growth promoting substance found widely in plants. It was first isolated from the fungus Gibberella. Gibberellin increases the rate of photosynthesis and helps in the elongation of cells. It promotes the flowering and germination of plants. With a general formula of Cl9H22O6, gibberellins are also produced by Azotobacter, Azospirillum, etc. | [Agricultural Uses]
Plant growth hormone, Plant regulator: Gibberellic acids (Gibberellins) are naturally occurring plant hormones that are used as plant growth
regulators to stimulate both cell division and elongation
that affects leaves and stems. Applications of this hormone also hastens plant maturation and seed germination.
Delayed harvesting of fruits, allowing them to grow larger.
Gibberellic acids are applied to growing field crops, small
fruits, grapes, vines and tree fruit, and ornamentals, shrubs
and vines. | [Trade name]
ACTIVOL®; BERELEX®; BRELLIN®;
BOLL-SET®; CEKUGIB®; CROP BOOSTER®;
CYTOPLEX HMS®, DYNOGEN®; FALGRO®;
FLORALTONE® (with 2,3,5-triiodobenzoic acid);
FLORGIB®; FOLI-ZYME®; GIBBEX®; GIBBERELLIN
A 3 ®; GIBBERELLIN X®; GIBBREL®; GIBGRO®;
GIB-SOL®; GIB-TABS®; GIBRESCOL®; GROCEL®;
KALGIBB®; MAXON®; N-LARGE®; NOVAGIB®;
PGR-IV®; PRO-GIBB®; REGULEX®; RELEASE®;
RELAX®; RYZUP®; STIMULATE®; VIGOR® | [Biochem/physiol Actions]
Phytohormone that controls important aspects of plant growth: germination, elongation, and flowering. The effects are mediated by inducing degradation of the DELLA repressor protein, and probably other minor signalling pathways. | [Purification Methods]
It crystallises from EtOAc, EtOAc/pet ether, MeOH/pet ether or Me2CO/pet ether. [Cross J Chem Soc 3022 1960, Beilstein 18 III/IV 6533.] | [Toxicity evaluation]
75 ppm of Gibberellic acid (GA3) in drinking water was continuously administered orally to rats (Sprague-Dawley albino) ad libitum for 50 days. The lipid peroxidation end product MDA significantly increased in the lungs, heart, and kidney of rats treated with GA3 without significant change in the spleen. Antioxidant enzyme activities such as SOD significantly increased in the lungs and stomach and decreased in the spleen and heart tissues of rats treated with GA3[4]. |
| Safety Data | Back Directory | [Hazard Codes ]
Xi | [Risk Statements ]
36 | [Safety Statements ]
26-36-24/25 | [RIDADR ]
None [88] Not regulated. | [WGK Germany ]
3
| [RTECS ]
LY8990000
| [F ]
10 | [TSCA ]
Yes | [HS Code ]
29322980 | [Safety Profile]
Mildly toxic by
ingestion. Questionable carcinogen with
experimental tumorigenic data. hlutation
data reported. When heated to
decomposition it emits acrid smoke and
irritating fumes. | [Hazardous Substances Data]
77-06-5(Hazardous Substances Data) | [Toxicity]
LD50 orally in Rabbit: 6300 mg/kg LD50 dermal Rabbit > 2000 mg/kg |
| Questions and Answers (Q&A) | Back Directory | [Description]
Gibberellic acid (GA) is a tetracyclic di-terpenoid compound. It is one of the major hormones that can be synthesized in plants and fungi. It has various kinds of physiological effects including stimulating seed germination, inducing mitotic division of the leaves, triggering transitions from meristem to shoot growth, vegetative to flowering, determining sex expression and grain development through crosstalk with many environmental signals such as light, temperature and water. In lab and greenhouse, it can be used to trigger germination of seeds which has previously remained dormant. It can also used to induce the production of larger amount and bigger grapes, boosting the grape industry. In industry, GA can be produced from Solid-state Fermentation (SSF) which can have relatively high yield.
| [References]
https://en.wikipedia.org/wiki/Gibberellic_acid
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4002599/
|
|
|