| Identification | More | [Name]
Antimony | [CAS]
7440-36-0 | [Synonyms]
ci77050
Stibium
Antymon
SB000070
SB007906
SB004700
SB000190
SB000140
SB000030
SB000120
SB007902
SB007910
SB000160
SB000300
ANTIMONY
c.i.77050
C.I. 77050
highpurity
Antimony bar
Antimony(Sb)
ANTIMONY, ROD
99.999%, SHOT
antimonyblack
Grey antimony
ANTIMONY SHOT
ANTIMONY METAL
Antimony black
AntiMony power
antimony ingot
Antimony, lump
Pure Tin powder
antymon(polish)
Thermoguard CPA
ANTIMONY POWDER
Antimony Pieces
antimony,regulus
ANTIMONY, 99.99%
ANTIMONY, 99.999%
AntiMony solution
ANTIMONY,METALLIC
ANTIMONY STANDARD
Antimony, regulus
regulusofantimony
ANTIMONYMETAL,LUMP
antimony,elemental
Antimonylumprandom
Antimonypowdermesh
Antimonyrod(99.8%)
AntimonyshotNmmdia
AntimonypowderNmesh
Antimony, Low Oxide
Antimony Shot 1-3 mm
ANTIMONYMETAL,POWDER
antimoineelementaire
ANTIMONY AA STANDARD
Antimony metal 99+ %
ANTIMONY METAL LUMPS
AntiMony shot,1-10MM.
ANTIMONY metal ingots
Antimonyshot(99.999%)
ANTIMONY ICP STANDARD
ANTIMONY LUMPS/POWDER
Antimonybar(99.999+%)
Antimonypowder(99.5%)
AntimonyshotNmmanddown
ANTIMONY 99.0%-99.999%
Antimonyshot(99.9999%)
Antimony, p.a., 0.15 mm
ANTIMONY: 99.999%, SHOT
AntimonyMetalLumps99.5%
Antimony, powder, 98.5%
antimony for high pmjty
ANTIMONY 99.999%, PIECES
Antimony ingots, 99.999%
Antimony(Metal)Powder99%
ANTIMONY, POWDER, 99.999%
ANTIMONY, PIECES, 99.999%
ANTIMONY ICP/DCP STANDARD
Antimony, powder, 0.15 mm
MERCURY 10,000PPM FOR ICP
antimony powder, -100 mesh
antimony powder, -325 mesh
ANTIMONY STANDARD SOLUTION
Antimony solution 1000 ppm
EUGON LT100 BROTH TUBE 9 ML
ANTIMONY (METAL) POWDER 99%
ANTIMONY METAL, HIGH PURITY
AntimonyrodNmmdiaxmmlonggmm
Antimony, shot, 2 mm & down
Antimony solution 10 000 ppm
Antimony Powder < 250 micron
Antimony Shot, 2mm And Down,
Antimony shot, 6 mm, 99.999%
ANTIMONY IMPLANTED IN SILICON
ANTIMONY, SHOT, 1-2MM, 99.999%
ANTIMONY: 99.6%, POWDER, -1 MM
Antimony pieces, ≤10 mm, 99.5%
Antimony, powder, 0.15 mm, 99+%
ANTIMONY, AAS STANDARD SOLUTION
ANTIMONY, 99+%, POWDER, 0.15 MM
Antimony, granulated, extra pure
ANTIMONY AA STANDARD CONCENTRATE
ANTIMONY SINGLE ELEMENT STANDARD
Antimony, lump, random, 99.9999%
ANTIMONY, LUMPS AND POWDER, 99.8%
ANTIMONY METALLO-ORGANIC STANDARD
ANTIMONY PLASMA EMISSION STANDARD
Antimony, shot 2mm & down 99.999%
Silicon phosphide single crystals
ANTIMONY, PLASMA STANDARD SOLUTION
ANTIMONY, POWDER, -100 MESH, 99.5%
ANTIMONY AA SINGLE ELEMENT STANDARD
ANTIMONY ATOMIC ABSORPTION STANDARD
Antimony powder, -200 mesh, 99.999%
Antimony powder, ~325 mesh, 99.99%+
ANTIMONY, SHOT, -4+20 MESH, 99.9998%
ANTIMONY, POWDER, -100 MESH, 99.995%
Antimony Shot, 2mm And Down, 99.999%
ANTIMONY POWDER FOR ANALYSIS PARTICLE
ANTIMONY ATOMIC SPECTROSCOPY STANDARD
Antimony rod, 12.7mm (0.500 in.) dia.
Antimony AA Standard,1000 ppm in HNO3
ANTIMONY, OIL BASED STANDARD SOLUTION
ANTIMONY SINGLE ELEMENT PLASMA STANDARD
ANTIMONY STANDARD SOLUTION TRACEABLE TO
ANTIMONY ICP STANDARD TRACEABLE TO SRM F
antimonyatomicabsorptionstandardsolution
ANTIMONY SINGLE ELEMENT STANDARD ICP-AES
Antimony shot, 1 to 3mm (0.04 to 0.1 in.)
Antimony shot, spherical, 1-5 mm, 99.999%
Antimony, AAS standard solution, Specpure?
ANTIMONY, SHOT, 1-5 MM, LOW OXIDE, 99.999%
Antimony rod, 50mm, diameter 4.7mm, 99.999%
Antimony AAS standard solution, Sb 1000μg/mL
Antimony, plasma standard solution, Specpure?
ANTIMONY PLASMA EMISSION SPECTROSCOPY STANDARD
Antimony powder, -325 mesh, 99.5% (metals basis)
Antimony rod, 10 to 12mm (0.39 to 0.47 in.) dia.
AntiMony powder, -200 Mesh, 99.5% (Metals basis)
Antimony powder, -100 mesh, 99.5% (metals basis)
ANTIMONY METAL POWDER extrapure min 99% -200 mesh
Antimony Oil based standard solution, Sb 1000μg/g
Antimony, Oil based standard solution, Sb 5000μg/g
Antimony, plasma standard solution, Sb 10,000μg/mL
Antimony, Reference Standard Solution, 1000ppm ±1%
ANTIMONY ATOMIC ABSORPTION SINGLE ELEMENT STANDARD
Standard solution for the determination of antimony
AntiMony powder, -100 Mesh, 99.5% trace Metals basis
ANTIMONY, SHOT, 1-5 MM, LOW OXIDE, 99.999% METALS BASIS
Antimony shot, 6mm (0.2in) & down, 99.999% (metals basis)
Antimony shot, 6.35mm (0.25in) & down, 99% (metals basis)
Antimony ingot, dia × length 18-20 mm × 110 mm, 99.99999%
Standard solution for determination of antimony impurities
Antimony rod, 12.7mm (0.50 in.) dia. x 10cm (3.9 in.) long
Antimony, AAS standard solution, Specpure(R), Sb 1000μg/ml
Antimony lump, 1.25cm (0.49in) & down, 99.5% (metals basis)
Antimony pieces, 5mm (0.2in) & down, 99.9999% (metals basis)
Antimony, plasma standard solution, Specpure(R), Sb 1000μg/ml
Antimony, plasma standard solution, Specpure(R), Sb 10,000μg/ml
Antimony, Oil based standard solution, Specpure(R), Sb 1000μg/g
Antimony, Oil based standard solution, Specpure(R), Sb 5000μg/g
ANTIMONY, LUMPS/POWDERANTIMONY, LUMPS/POWDERANTIMONY, LUMPS/POWDER
Antimony lump, polycrystalline, Puratronic�, 99.9999% (metals basis)
Antimony lump, polycrystalline, Puratronic(R), 99.9999% (metals basis)
Antimony shot, 1-3mm (0.04-0.1in), Puratronic�, 99.9999% (metals basis)
Antimony shot, 1.5-5mm (0.06-0.2in), Puratronic�, 99.9999% (metals basis)
Antimony shot, 1-3mm (0.04-0.1in), Puratronic(R), 99.9999% (metals basis)
Antimony shot, 1.5-5mm (0.06-0.2in), Puratronic(R), 99.9999% (metals basis)
Antimony broken rod, 12.7mm (0.50in) dia x various lengths, 99.8% (metals basis)
Antimony shot, spherical, 1-5mm (0.04-0.20in), low oxide, 99.999% (metals basis)
ANTIMONY, 99.999%, PIECESANTIMONY, 99.999%, PIECESANTIMONY, 99.999%, PIECESANTIMONY, 99.999%, PIECES | [EINECS(EC#)]
231-146-5 | [Molecular Formula]
Sb | [MDL Number]
MFCD00134030 | [Molecular Weight]
121.76 | [MOL File]
7440-36-0.mol |
| Chemical Properties | Back Directory | [Appearance]
Antimony is a silvery-white, lustrous, hard,
brittle metal; scale-like crystals, or dark gray lustrous powder | [Melting point ]
630 °C (lit.) | [Boiling point ]
1635 °C (lit.) | [density ]
6.69 g/mL at 25 °C(lit.)
| [Fp ]
1380°C | [storage temp. ]
Store at +15°C to +25°C. | [solubility ]
H2O: soluble
| [form ]
powder
| [color ]
Silver-gray | [Specific Gravity]
6.684 | [Flame Color]
Pale green | [Water Solubility ]
INSOLUBLE | [Merck ]
13,698 | [Exposure limits]
ACGIH: TWA 2 ppm; STEL 4 ppm
OSHA: TWA 2 ppm(5 mg/m3)
NIOSH: IDLH 25 ppm; TWA 2 ppm(5 mg/m3); STEL 4 ppm(10 mg/m3) | [History]
Antimony was recognized
in compounds by the ancients and was known as a metal
at the beginning of the 17th century and possibly much earlier.
It is not abundant, but is found in over 100 mineral species. It
is sometimes found native, but more frequently as the sulfide,
stibnite (Sb2S3); it is also found as antimonides of the heavy
metals, and as oxides. It is extracted from the sulfide by roasting
to the oxide, which is reduced by salt and scrap iron; from
its oxides it is also prepared by reduction with carbon. Two
allotropic forms of antimony exist: the normal stable, metallic
form, and the amorphous gray form. The so-called explosive
antimony is an ill-defined material always containing an appreciable
amount of halogen; therefore, it no longer warrants
consideration as a separate allotrope. The yellow form, obtained
by oxidation of stibine, SbH3, is probably impure, and
is not a distinct form. Natural antimony is made of two stable
isotopes, 121Sb and 123Sb. Forty-five other radioactive isotopes
and isomers are now recognized. Metallic antimony is an extremely
brittle metal of a flaky, crystalline texture. It is bluish
white and has a metallic luster. It is not acted on by air at room
temperature, but burns brilliantly when heated with the formation
of white fumes of Sb203. It is a poor conductor of heat
and electricity, and has a hardness of 3 to 3.5. Antimony, available
commercially with a purity of 99.999 + %, is finding use
in semiconductor technology for making infrared detectors,
diodes, and Hall-effect devices. Commercial-grade antimony
is widely used in alloys with percentages ranging from 1 to 20.
It greatly increases the hardness and mechanical strength of
lead. Batteries, antifriction alloys, type metal, small arms and
tracer bullets, cable sheathing, and minor products use about
half the metal produced. Compounds taking up the other
half are oxides, sulfides, sodium antimonate, and antimony
trichloride. These are used in manufacturing flame-proofing
compounds, paints, ceramic enamels, glass, and pottery.
Tartar emetic (hydrated potassium antimonyl tartrate) has
been used in medicine. Antimony and many of its compounds
are toxic. Antimony costs about $1.30/kg for the commercial
metal or about $12/g (99.999%). | [CAS DataBase Reference]
7440-36-0(CAS DataBase Reference) | [NIST Chemistry Reference]
Antimony(7440-36-0) | [EPA Substance Registry System]
7440-36-0(EPA Substance) |
| Hazard Information | Back Directory | [Chemical Properties]
Antimony is a silvery-white metal found in the earth’s crust. It is insoluble in hot or
cold water, but soluble in hot concentrated sulfuric acid and hot nitric acid, and reacts
with oxidizing acids and halogens (fl uorine, chlorine, or bromine). It does not react with
water at room temperature, but will ignite and burn in air at higher temperatures. Ores
of antimony are mined and later mixed with other metals to form antimony alloys,
which are used in lead storage batteries, solder, sheet and pipe metal, bearings, castings, and pewter. Antimony oxide is added to textiles and plastics to prevent them from
catching fi re. It is also used in paints, ceramics, and fi reworks, and as enamels for plastics, metal, and glass. Antimony is alloyed with other metals, such as lead, to increase
its hardness and strength; its primary use is in antimonial lead, which is used in grid
metal for lead acid storage batteries. Antimony salts are used in the treatment of leishmaniasis and schistosomiasis. | [General Description]
A silvery or gray solid in the form of dust. Denser than water and insoluble in water. Toxic by inhalation and by ingestion. May burn and emit toxic fumes if heated or exposed to flames. Used to make electric storage batteries and semiconductors. | [Reactivity Profile]
ANTIMONY is spontaneously flammable in fluorine, chlorine, and bromine. With iodine, the reaction produces heat, which can cause flame or even an explosion if the quantities are great enough [Mellor 9:379 1946-47]. Even at 10° C. bromine trifluoride reacts with antimony incandescently. Bromine trifluoride reacts similarly with arsenic, boron, bromine, iodine, phosphorus, and sulfur [Mellor 2:113 1946-47]. Bromoazide explodes on contact with antimony, arsenic, phosphorus, silver foil, or sodium. ANTIMONY POWDER(7440-36-0) is very shock sensitive. Explosions of chloric acid have been due to the formation of unstable compounds with antimony, bismuth, ammonia, and organic matter [Chem. Abst. 46:2805e 1952]. The reaction of finely divided antimony and nitric acid can be violent [Pascal 10:504 1931-34]. Powdered antimony mixed with potassium nitrate explodes when heated [Mellor 9:282 1946-47]. When antimony or arsenic and solid potassium permanganate are ground together, the metals ignite [Mellor 12:322 1946-47]. Sodium peroxide oxidizes antimony, arsenic, copper, potassium, tin, and zinc with incandescence [Mellor 2:490-93 1946-47]. | [Air & Water Reactions]
Insoluble in water. | [Hazard]
Use with adequate ventilation. Soluble salts
are toxic.
| [Health Hazard]
Oxides from metallic fires are a severe health hazard. Inhalation or contact with substance or decomposition products may cause severe injury or death. Fire may produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may cause pollution. | [Health Hazard]
The toxicity of antimony compounds is comparable to that of arsenic, but as antimony
compounds are hardly absorbed in the gastrointestinal tract, there is less hazard of acute
poisoning. In addition, antimony compounds often cause vomiting, thus being removed
from the organism. Chronic poisoning may result in damage to the liver, kidneys, and
even the heart and the circulatory system. The symptoms differ among the compounds.
Stibine accumulates in fatty tissue. Exposures to antimony and its compounds cause
poisoning and toxicity to the worker with symptoms that include, but are not limited to,
irritation to eyes, skin, nose, and throat, ulceration of nasal septum and larynx, dermatitis as characterized by antimony spots, cough, dizziness, seizures, headache, anorexia,
nausea, vomiting, diarrhea, stomach cramps, bloody stools, insomnia, inability to smell
properly, metallic taste, cardiovascular disturbances, pulmonary edema, pharyngitis,
tracheitis, heart and lung damage, pneumoconiosis, slow and shallow respiration, coma,
and death. Antimony fumes and dusts inhaled by occupational workers are associated
with the development of benign tumors of the lungs, dermatitis and, less commonly,
effects on the heart and kidneys. Laboratory animals exposed to antimony by inhalation or ingestion exhibit effects similar to those noted in humans. Antimony can be
measured in the urine, feces, and blood. To date, little is known about the environmental
risks involved. Water pollution seldom occurs because of the low solubility of most compounds. Extreme caution should be taken when coming into direct contact with antimony compounds. | [Potential Exposure]
Exposure to antimony may occur during
mining, smelting or refining; alloy and abrasive manufacture;
and typesetting in printing. Antimony is widely
used in the production of alloys, imparting increased hardness,
mechanical strength, corrosion resistance, and a low
coefficient of friction. Some of the important alloys are
Babbitt, pewter, white metal, Britannia metal and bearing
metal (which are used in bearing shells), printing-type,
metal, storage battery plates, cable sheathing, solder, ornamental
castings, and ammunition. Pure antimony compounds
are used as abrasives, pigments, flame-proofing
compounds, plasticizers, and catalysts in organic synthesis;
they are also used in the manufacture of tartar emetic,
paints, lacquers, glass, pottery, enamels, glazes, pharmaceuticals,
pyrotechnics, matches, and explosives. In addition,
they are used in dyeing, for blueing steel; and in coloring
aluminum pewter; and zinc. A highly toxic gas, stibine,
may be released from the metal under certain conditions. | [Fire Hazard]
May react violently or explosively on contact with water. Some are transported in flammable liquids. May be ignited by friction, heat, sparks or flames. Some of these materials will burn with intense heat. Dusts or fumes may form explosive mixtures in air. Containers may explode when heated. May re-ignite after fire is extinguished. | [First aid]
If this chemical gets into the eyes, remove any
contact lenses at once and irrigate immediately for at least
15 minutes, occasionally lifting upper and lower lids. Seek
medical attention immediately. If this chemical contacts
the skin, remove contaminated clothing and wash immediately
with soap and water. Seek medical attention immediately.
If this chemical has been inhaled, remove from
exposure, begin rescue breathing (using universal precautions,
including resuscitation mask) if breathing has
stopped and CPR if heart action has stopped. Transfer
promptly to a medical facility. When this chemical has
been swallowed, get medical attention. Give large quantities
of water and induce vomiting. Do not make an unconscious
person vomit. | [Shipping]
UN2871 Antimony powder, Hazard Class: 6.1;
Labels: 6.1-Poisonous materials. | [Incompatibilities]
Pyrophoric. Finely dispersed powder
may form explosive mixture in air. Strong oxidizers; strong
acids , produce a violent
reaction, and deadly stibine gas (antimony hydride). Heat
forms stibine gas. Mixtures with nitrates or halogenated
compounds may cause combustion. Forms an explosive
mixture with chloric and perchloric acid. Note: Stibine is
formed when antimony is exposed to nascent (freshly
formed) hydrogen. | [Waste Disposal]
Recovery and recycling is an
option to disposal which should be considered for scrap antimony
and spent catalysts containing antimony. Dissolve
spilled material in minimum amount of concentrated HCl.
Add water, until white precipitate appears. Then acidify to dissolve
again. Saturate with H2S. Filter, wash and dry
the precipitate and return to supplier. Consult with environmental
regulatory agencies for guidance on acceptable disposal
practices. Generators of waste containing this contaminant
(≥100 kg/mo) must conform with EPA regulations governing
storage, transportation, treatment, and waste disposal. | [Physical properties]
Physically, antimony’s properties are related to sulfur and some of the nonmetals, butchemically, its properties are related to metals. It behaves like a metal and is often found innature along with other metals. In its pure form it is rather hard and brittle with a grayishcrystal structure. | [Isotopes]
There are 53 isotopes of antimony. They range from Sb-103 to Sb-139 (a fewhave two forms). Their half-lives range from 150 nanoseconds to 2.7 years. The twostable isotopes of antimony and their contribution to the natural abundance of antimonyon Earth are as follows: Sb-121 = 57.21% and Sb-123 = 42.79%. | [Origin of Name]
The element’s name comes from the Greek words anti and minos,
which mean “not alone,” and antimony’s symbol (Sb) is derived from the name for its
ancient source mineral, stibnium. | [Occurrence]
Although antimony is not a rare metal, it is not well known, despite having been knownand used for many centuries. It is the 63rd most abundant element on Earth, and it occursmainly as sulfide ores or in combination with the ores of other metals. The ore that is theprimary source of antimony is the mineral stibnite (antimony sulfide, Sb2S3). Antimony is alsofound in copper, silver, and lead ores. Breithauptite (NiSb) and ullmanite (NiSbS) are twoores containing nickel. Dicrasite (Ag2Sb) and pyrargyrite (Ag3SbS3) are silver ores containingsome antimony. | [Characteristics]
There are two allotropes of antimony. The native metallic form is one allotrope, and theother allotrope is an amorphous grayish form. Antimony is a true metalloid that is brittle witha low melting point. And similar to nonmetals, it is a poor conductor of heat and electricity.
Antimony is unique in that when it solidifies from a molten liquid state to a solid state, itexpands, which is just the opposite of most metals. This is useful in making some typesettingcastings in which the expansion assures an accurate reproduction of the letter mold. | [Application]
It is important as wire materials of the thermocouples for spectroscopic measurements. The alloy with other metal is used because the material is fragile. | [Definition]
antimony: Symbol Sb. An elementbelonging to group 15 (formerly VB)of the periodic table; a.n. 51; r.a.m.121.75; r.d. 6.68; m.p. 630.5°C; b.p.1750°C. Antimony has several allotropes.The stable form is a bluishwhitemetal. Yellow antimony andblack antimony are unstable nonmetallicallotropes made at low temperatures.The main source isstibnite (Sb2S3), from which antimonyis extracted by reduction withiron metal or by roasting (to give theoxide) followed by reduction withcarbon and sodium carbonate. Themain use of the metal is as an alloyingagent in lead-accumulator plates,type metals, bearing alloys, solders,Britannia metal, and pewter. It is alsoan agent for producing pearlitic castiron. Its compounds are used inflame-proofing, paints, ceramics,enamels, glass dyestuffs, and rubbertechnology. The element will burn inair but is unaffected by water or diluteacids. It is attacked by oxidizingacids and by halogens. It was first reportedby Tholden in 1450. | [Manufacturing Process]
Antimony metal is recovered from ore primarily by pyrometallurgical techniques. Either antimony(III) sulfide is converted into the oxide, which is then reduced, or the ore is partially roasted and allowed to react with sulfide to form the metal and sulfur dioxide. Sulfide ores with antimony contents between 5 and 25% are roasted to give volatile Sb2O3, which is reduced directly to the metal. In many smelters mixed oxide – sulfide ores are pro�cessed in water-jacketed furnaces together with recycled material and byproducts containing antimony. Reverberatory furnaces are used mostly for reducing rich oxide materials.
| [Pharmaceutical Applications]
Antimony presents itself
in a metallic grey form. Antimony is obtained from stibnite (Sb2S3) after reduction with iron. | [Industrial uses]
Antimony is a bluish-white metal, symbol Sb,with a crystalline scalelike structure that exhibitspoor electrical and heat conductivity. It isbrittle and easily reduced to powder. It is neithermalleable nor ductile and is used only in alloysor in its chemical compounds. Like arsenic andbismuth, it is sometimes referred to as a metalloid,but in mineralogy it is called a semimetal.The element is available commercially in99.999+% purity and is finding increasing usein semiconductor technology.
Antimony is produced either by roasting thesulfide with iron, or by roasting the sulfide andreducing the sublimate of Sb4O6 thus producedwith carbon; high-purity antimony is producedby electrolytic refining. Antimony is one of thefew elements that exhibits the unique propertyof expanding on solidification. Antimony isordinarily stable and not readily attacked by airor moisture. Under controlled conditions it willreact with O2 to form oxides. The chief uses ofantimony are in alloys, particularly for hardeninglead-base alloys.
Antimony imparts hardness and a smoothsurface to soft-metal alloys, and alloys containingantimony expand on cooling, thus reproducingthe fine details of the mold. This propertymakes it valuable for type metals. When alloyedwith lead, tin, and copper, it forms the babbittmetals used for machinery bearings. It is alsomuch used in white alloys for pewter utensils.Its compounds are used widely for pigments. | [Carcinogenicity]
Existing experimental data suggest that antimony may be an animal carcinogen, but there is
lack of data on the possible carcinogenic properties of antimony and antimony compounds for human exposures. The ACGIH refers to unpublished data on a large antimony smelter in the United Kingdom in the 1960s where workers were exposed to antimony trioxide ranging from 0.5 to 40mg/m3. The data may indicate increased mortality in lung cancer among the heavily exposed workers, but the workers were also exposed to zirconium making the data cited dif?cult to interpret. | [Environmental Fate]
The toxicity of Sb is a function of the water solubility and the
oxidation state of the Sb species under consideration. Antimony(
III) is generallymore toxic than antimony(V) and inorganic
forms are thought to be more toxic than organic forms. Stibane
gas (SbH3) when inhaled is the most toxic. Antimony toxicity
often parallels that of arsenic, although antimony salts are less
readily absorbed than arsenic. It is presumed that antimony, like
arsenic, complexes with sulfhydryl groups of essential enzymes
and other proteins. By analogy, antimony can uncouple oxidative
phosphorylation, which would inhibit the production of energy
necessary for cellular functions. Antimony’s trivalent compounds
are more toxic than its pentavalent compounds. | [storage]
Color Code—Blue: Health Hazard/Poison: Store ina secure poison location. Prior to working with this chemicalyou should be trained on its proper handling and storage.Store in tightly closed containers in a cool, well-ventilatedarea away from oxidizers, halogens, strong acids, and heat.Sources of ignition, such as smoking and open flames, areprohibited where this chemical is used, handled, or stored ina manner that could create a potential fire or explosion hazard. Contact with acids forms deadly stibine gas. Beforeentering confined space where this chemical may be present,check to make sure that an explosive concentration does notexist. | [Structure and conformation]
The space lattice of most stable metallic antimony (often called gray antimony) belongs to the hexagonal system, and its arsenic type structure (two atoms within a unit cell) has a lattice constant of a=0.449762 nm, a=57°6.6', u=0.233. Black antimony and yellow antimony are known, but these are unstable and transform to metallic antimony
| [Toxicity evaluation]
Antimony is found naturally in the Earth’s crust and can be
released into the environment as windblown dust or sea spray
or from volcanic eruptions or forest fires. However, the emission
of antimony into the environment is overwhelmingly the
result of human activity, with the emission of antimony
trioxide, tetroxide, and pentoxide forms being the most
significant. Antimony trioxide is emitted as a result of coal
burning, or with fly ash when antimony-containing ores are
smelted. Humans are exposed to low amounts of antimony
from the air, drinking water, and food contaminated with
soil. Antimony concentration in the atmosphere is thought to
be 1.4–55 ng m-3. The more water soluble forms of antimony
are very mobile in aqueous media while the less soluble forms
of antimony are found attached to particles of soil, clay, and
sediment in rivers and lakes. The concentration of antimony in
the Pacific Ocean was found to be 0.2 mg l-1 and in the Rhine
river at 0.1 μg l-1. The trivalent state of antimony is the form
most often released by anthropogenic activities. In terms of soil
concentrations, it was reported by a US Geological Survey to be
less than 1–8 ppm in soil, with an average of 0.48 ppm. Studies
have estimated an exposure of less than 5 mg day�1 on average
from food and water and appears to be significantly higher
than exposure by inhalation.
| [Toxics Screening Level]
The initial threshold screening level (ITSL) for antimony is 0.2 μg/m3 (annual averaging time). |
| Safety Data | Back Directory | [Hazard Codes ]
N,Xn,Xi | [Risk Statements ]
R34:Causes burns.
R51/53:Toxic to aquatic organisms, may cause long-term adverse effects in the aquatic environment .
R20/22:Harmful by inhalation and if swallowed .
R36/37/38:Irritating to eyes, respiratory system and skin .
R36/38:Irritating to eyes and skin . | [Safety Statements ]
S60:This material and/or its container must be disposed of as hazardous waste .
S61:Avoid release to the environment. Refer to special instructions safety data sheet .
S36/37/39:Wear suitable protective clothing, gloves and eye/face protection .
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice . | [OEB]
C | [OEL]
TWA: 0.5 mg/m3 [*Note: The REL also applies to other antimony compounds (as Sb).] | [RIDADR ]
UN 3264 8/PG 2
| [WGK Germany ]
2
| [RTECS ]
CC4025000
| [TSCA ]
Yes | [HazardClass ]
6.1 | [PackingGroup ]
III | [HS Code ]
81101000 | [Precautions]
Antimony trioxide is incompatible with bromine trifl uoride, strong acids, strong bases,
reducing agents, perchloric acid, and chlorinated rubber. The release of the deadly gas,
stibine, and its inhalation cause adverse effects on the respiratory, gastrointestinal, and
cardiovascular systems. Workers must wear impervious protective clothing, including
boots, gloves, laboratory coat, apron or coveralls, as appropriate, to prevent skin contact. | [Safety Profile]
An experimental poison
by intraperitoneal route. Questionable
carcinogen with experimental carcinogenic
data. Moderate fire and explosion hazard in
the forms of dust and vapor when exposed
to heat or flame. See also POWDERED
METALS. When heated or on contact with
acid it emits toxic fumes of SbH3.
Electrolysis of acid sulfides and stirred Sb
halide yields explosive Sb. It can react
violently with NH4NO3, halogens, BrN3,
BrF3, HClO3, Cl0, ClF3, HNO3, m03,
KMn04, K2O2, NaNO3, oxidants. | [Hazardous Substances Data]
7440-36-0(Hazardous Substances Data) | [Toxicity]
LD50 in rats, guinea pigs (mg Sb/100 g): 10.0, 15.0 i.p. (Bradley, Fredrick) | [IDLA]
50 mg Sb/m3 |
| Questions And Answer | Back Directory | [Description]
Antimony occurs in nature primarily in the mineral stibnite, and also in several other ores, such as valentinite, senarmontite, cervantite, kermasite, livingstonite, and jamisonite. It is also found in lead scraps from batteries.
Antimony alloys have many commercial applications. The metal makes its alloys hard and stiff and imparts resistance to corrosion. Such alloys are used in battery grids and parts, tank linings, pipes and pumps. The lead plates in the lead storage batteries constitute 94% lead and 6% antimony. Babbit metal, an alloy of antimony, tin, and copper is used to make antifriction machine bearings. Alloys made from very high purity grade antimony with indium, gallium and bismuth are used as infrared detectors, diodes, hall effect devices and thermoelectric coolers.
| [Chemical Properties]
A natural element, antimony (symbol Sb; CASRN 7440-36-0) occurs in valence states of 3, 5, and -3 and has both metallic and nonmetallic properties. It is commercially available as a silver white lustrous solid metal or a dark gray powder (HSDB, 2005; Budavari, 1989). The amount in the earth’s crust is <1 parts per million (ppm); its most common ore is stibnite (CASRN 1345-04-6). Antimony trisulfide (symbol Sb2S3; CASRN 1317-86-8), is a chemical form of antimony (Beliles, 1979). In the soils of the conterminous United States, it occurs at a geometrical mean of 0.48 ppm (Shacklett and Boerngen, 1984).

Because antimony is a group VA element, it has many of the same chemical and toxicological properties as arsenic and lead. For example, the toxicity of pentavalent antimony compounds is less than that of trivalent compounds (DeWolff and Edelbroek, 1994). The suggested descending order of toxicity is metalloid antimony (particularly stibine gas), the trisulfide, the pentasulfide, the trioxide, and the pentoxide.
| [History]
Antimony has been an important mineral through much of human history. For example, the ancient Egyptians and early Hindus used stibnite (Sb2S3), which is the major ore mineral for antimony, to produce black eye makeup as early as about 3100 B.C. Medieval alchemists thought that antimony could be used to convert lead into gold. Today, antimony is used in lead-acid storage batteries for backup power and transportation; in chemicals, ceramics, and glass; in flameretardant materials; and in heat stabilizers and plastics. | [Uses]
Antimony's leading use is as a fire retardant in safety equipment and in household goods, such as mattresses.
Antimony has more uses of a direct military character than other members of the strategic group and probably more important uses than any of the others except mercury.Antimony is a hardening agent in metals used in ball bearings, bullets capable of penetrating armor plate, and lead shot. It helps to strengthen cable sheaths, chemical pumps, foils, plumbing fixtures and pipes, roofing sheets, and tank linings. During World War II, the fireproofing compound antimony trichloride (SbCl3) saved the lives of many American troops when it was applied to tents and vehicle covers. In a fire, antimony and chlorine recombine to form unstable compounds that remove oxygen from the air, smothering the flames (Gibson, 1998; Eyi, 2012).
| [Production Methods]
Antimony is obtained from its ores, stibnite, Sb2S3 or tetrahedrite, 3Cu2S . Sb2S3. The metal is recovered from high-grade stibnite by reduction with iron:
Sb2S3 + 3 Fe → 2 Sb + 3 FeS
Alternatively, low-grade stibnite ore is converted to its oxide which is then reduced with carbon. Tetrahedrite may be treated with sodium sulfide solution. The solution containing thioantimonate formed is then electrolyzed in a diaphragm cell using a steel cathode and lead anode. The metal is further refined by oxidation or electrorefining process.
Sb may be made in the laboratory by reduction of antimony pentoxide with potassium cyanide.
| [Reduction from Oxides]
The oxides from volatilizing roasting, as well as other oxidized antimony materials, are reduced to the metal with carbonaceous materials. Occasionally oxide ores containing more than 50 % antimony are finely ground and also reduced. Sometimes the oxide must be treated beforehand to remove arsenic, but improved roasting techniques frequently make this unnecessary. The amount of carbon required for reduction depends on the composition of the oxide, ranging between 8 and 12 %. Pulverized charcoal, anthracite dust, or lean coke breeze are used.
Smelting of antimony oxide flue dust containing arsenic, such as is obtained by roasting ores, with sodium hydroxide produces metallic antimony.
2 Sb2O3 + 3 As2O3 + 18 NaOH→4 Sb+6 Na3AsO4+9 H2O
The oxides are reduced in shaft, reverberatory, or short rotary furnaces. All types of fur�naces require efficient precipitators and off-gas filters to remove volatilized antimony(III) oxide.
| [Antimony and Antimony Compounds]
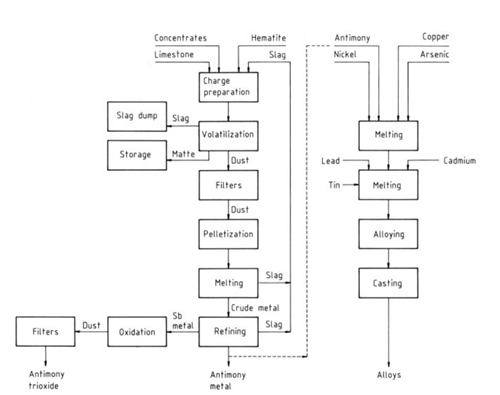 | [Hydrometallurgical Methods]
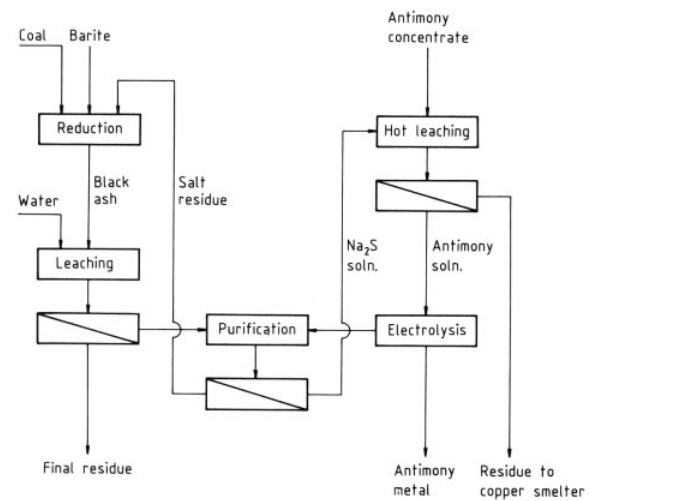
Antimony ores can be digested by alkalies. The mechanochemical leaching of antimony from Slovakian tetrahedrite concentrate in an attritor and the chemical leaching of the resulting solid residues were studied. The results indicate that recoveries of 52 – 100 % Sb, 60 – 85 % Hg and 50 – 96 % Ag can be achieved by using alkaline solutions of Na2S for leaching of Sb and Hg. The dissolution of Sb from a Columbian stibnite concentrate in basic solutions of Na2S·9H2O and NaOH gave a maximum Sb extraction of 94 % from a concentrate containing 30 % Sb with a mixture of 12.5% Na2S and 4.0 % NaOH.
| [Electrolysis Methods]
The antimony is recovered from the alkaline solution by electrolysis. The steps are as follows:
1) The antimony is batch leached with hot concentrated sodium sulfide solution from milled tetrahedrite concentrate to give a solution containing about 250 g Na2S and 50 g Sb per liter. The leaching time is 8 – 10 h at 100 – 103 ℃.
2) After the residue settles, the supernatant solution is decanted. The residue is recovered by two-stage filtration with a repulping step between the stages. The pregnant solution is fed to the electrolytic section through two anolyte and two catholyte tanks.
3) The solution is batch electrolyzed in banks of diaphragm cells. The antimony is deposited at mild-steel cathodes. The electrolytic section consists of 96 cells in series, arranged in cascades of six.
 | [Toxicokinetics]
Most antimony compounds, mainly those with poor water solubility, are absorbed only slowly from the gastrointestinal tract. Trivalent compounds especially tend to accumulate in the human body, because they are excreted very slowly via urine and feces. Antimony and its compounds react readily with mercapto groups in various cellular constituents, especially in enzymes, blocking their activity. After acute and chronic intoxications, the highest levels of antimony were found in lungs, liver and kidneys.
|
|