| Identification | Back Directory | [Name]
METHYLTRIOXORHENIUM(VII) | [CAS]
70197-13-6 | [Synonyms]
MTO
METHYLTRIOXORHENIUM
METHYLTRIOXORHENIUM(7)
METHYLRHENIUM TRIOXIDE
Methyltrioxorhenium(Ⅶ)
METHYLTRIOXORHENIUM(VII)
METHYLRHENIUM TRIOXIDE(VII)
Methylrhenium(VII) trioxide
Rhenium,methyltrioxo-,(T-4)-
Methyltrioxorhenium(VII),98%
MethyltrioxorheniuM(VII) (MTO)
Methylrhenium(VII) trioxide, MTO
MethyltrioxorheniuM (VII), 98% 100MG
MethyltrioxorheniuM (VII),98% CH3ReO3
MethyltrioxorheniuM(VII) Re 71.0-76.0 %
METHYLTRIOXORHENIUM(VII), 71.0%-76.0% RE
Methyltrioxorhenium(VII),MTO, Methylrhenium(VII) trioxide | [EINECS(EC#)]
-0 | [Molecular Formula]
CH3O3Re | [MDL Number]
MFCD00192332 | [MOL File]
70197-13-6.mol | [Molecular Weight]
249.24 |
| Chemical Properties | Back Directory | [Appearance]
white to blue-grey crystals | [Melting point ]
111 °C(lit.)
| [Boiling point ]
65°C 0mm | [density ]
d23 4.21 | [form ]
crystal | [color ]
colorless to pale-gray | [Water Solubility ]
Soluble in water. | [Sensitive ]
Moisture & Light Sensitive | [Merck ]
14,6130 | [BRN ]
3927932 |
| Hazard Information | Back Directory | [Chemical Properties]
white to blue-grey crystals | [Uses]
Potent catalytic oxidant for converting alkenes to epoxides in a variety of solvents and 2-methylnaphthalene to 2-methyl-1,4-naphthoquinone (Vitamin K3).
Catalytic oxidant used with urea hydrogen peroxide in an efficient conversion of imines to nitrones.
An effective antioxidant under various conditions. | [Uses]
Methyltrioxorhenium(VII) is used as a catalyst for various transformations such as olefin metathesis. It is associated with hydrogen peroxide and used as an oxidizing agent. It is used to prepare acid or ester from the corresponding terminalalkynes. It is a catalytic oxidant used with urea hydrogen peroxide to prepare nitrones. It reacts with 2-methylnaphthalene to get 2-methyl-1,4-naphthoquinone (Vitamin K3). Further, it catalyzes the reaction with aldehydes and diazoketones to get an alkene. In addition to this, it is used as an effective antioxidant under various conditions. |
| Questions And Answer | Back Directory | [Reactions]
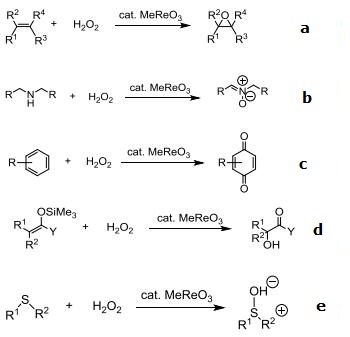
Catalyst used with H2O2 for oxidation of a variety of substrates.
(a) Alkenes
(b) Secondary amines
(c) Arenes
(d) Silyl enol ethers/Silyl ketene acetals
(e) Sulfides
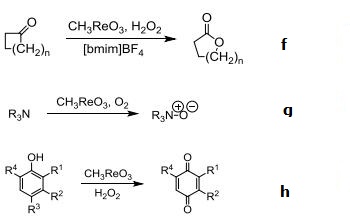
(f) Bayer-Villager-Type oxidation
(g) Amine oxidation
(h) Phenol oxidation
|
|
|