| Identification | More | [Name]
Noctone | [CAS]
66357-59-3 | [Synonyms]
N-[2-[[[5-[(DIMETHYLAMINO)METHYL]-2-FURANYL]METHYL]THIO]ETHYL]-N'-METHYL-2-NITRO-1,1-ETHANEDIAMINE HYDROCHLORIDE
N-[2-[[[-5-[(DIMETHYLAMINO)METHYL]-2-FURANYL]METHYL]THIO]ETHYL]-N'-METHYL-2-NITRO-1,1 ETHENEDIAMINE, HYDROCHLORIDE
n'-[2-[[5-(dimethylaminomethyl)-2-furyl]methylsulfanyl]ethyl]-n-methyl-2-nitro-ethene-1,1-diamine hydrochloride
N-[2-[5-[(DIMETHYLAMINO)METHYL]FURFURYLTHIO]ETHYL]-N'-METHYL-2-NITRO-1,1-ETHENEDIAMINE HYDROCHLORIDE
n-[2-[[5-[(dimethylamino)methyl]furfuryl]thio]ethyl]-n'-methyl-2-nitrovinylidenediamine hydrochloride
n-[2-[[5-[(dimethylamino)methyl]furfuryl]thio]ethyl]-n'-methyl-2-nitrovinylidenediamine monohydrochloride
noctone
RANITIDINE HCL
RANITIDINE HYDROCHLORIDE
ZANTAC
ah19065
ranidil
ranigast
Ranitidine hydrochloride USP24
Rantidine hydrochloride USP24
Ranitidine hydrochloride CP2000,USP24
Rantidine HCL
Ranitidine HCL BP2000/USP25
RANITIDINE HCL USP (FORM 1)
RANITIDINE HCL USP (FORM 2) | [EINECS(EC#)]
266-332-5 | [Molecular Formula]
C13H23ClN4O3S | [MDL Number]
MFCD00069339 | [Molecular Weight]
350.86 | [MOL File]
66357-59-3.mol |
| Chemical Properties | Back Directory | [Appearance]
Off-White Crystalline Solid | [Melting point ]
134°C (dec.) | [storage temp. ]
Hygroscopic, -20°C Freezer, Under Inert Atmosphere | [solubility ]
H2O: 1.8 mg/mL
| [form ]
solid
| [color ]
tan
| [Water Solubility ]
Soluble in water, 2-hydroxypropyl-beta-cyclodextrin, acetic acid, methanol, ethanol and dimethyl sulfoxide. Insoluble in chloroform. | [Sensitive ]
Hygroscopic | [Usage]
A histamine H2-receptor antagonist which inhibits gastric acid secretion. Antiulcerative. | [Merck ]
14,8110 | [BCS Class]
3/1 | [Stability:]
Hygroscopic | [Contact allergens]
Ranitidine, an H2-receptor antagonist, can cause contact
dermatitis within the pharmaceutical industry and
in health care workers, or may induce systemic drug
reactions in patients. | [CAS DataBase Reference]
66357-59-3(CAS DataBase Reference) |
| Hazard Information | Back Directory | [Chemical Properties]
Off-White Crystalline Solid | [Uses]
A histamine H2-receptor antagonist which inhibits gastric acid secretion. Antiulcerative. | [Uses]
antifungal | [Brand name]
Zantac (GlaxoSmithKline). | [Biological Activity]
ranitidine is a histamine h2-receptor antagonist that inhibits stomach acid production.ranitidine (zantac) is a histamine h2-receptor antagonist with ic50 of 3.3 ± 1.4 um. it inhibits stomach acid production. it is also used alongside fexofenadine and oth | [Biochem/physiol Actions]
H2 histamine receptor antagonist; anti-ulcer agent. | [storage]
Store at -20°C |
| Safety Data | Back Directory | [Hazard Codes ]
F,T | [Risk Statements ]
R20/21/22:Harmful by inhalation, in contact with skin and if swallowed . | [Safety Statements ]
S22:Do not breathe dust .
S24/25:Avoid contact with skin and eyes . | [WGK Germany ]
2
| [RTECS ]
KM6557000
| [HS Code ]
2932190002 |
| Questions And Answer | Back Directory | [Background and overview]
Ranitidine Hydrochloride (RHCl) is a kind of histamine H2 receptor antagonist. Since its listing in 1981, ranitidine hydrochloride has been widely used in nearly one hundred countries in the world, including China. It is clinically used for the treatment of duodenal ulcer, reflux esophagitis and Zollinger-Ellison syndrome. It is also used for the prevention of gastrointestinal bleeding caused by stress ulcer and recurrent hemorrhage of peptic ulcer. In recent decade, through the combination of ranitidine and other drugs, it has found that it has high efficiency and remarkable features in the treatment of Helicobacter pylori-positive duodenal ulcer, urticaria and post-cerebral hemorrhage stress ulcer with better efficacy than other similar drugs. Owing to its rapid effect, good potency and low price, ranitidine hydrochloride plays an important role in the anti-ulcer drug market today. Therefore, strict quality control plays important roles in guiding patients with reasonable, safe medication.
| [Pharmacological effects]
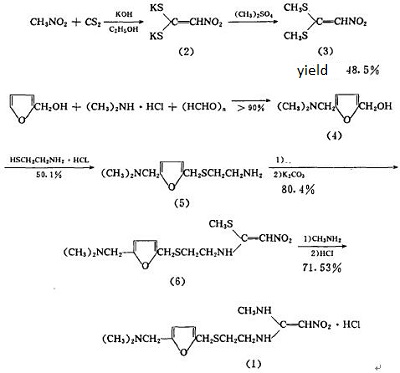
This product is a H2 receptor inhibitor, being capable of inhibiting the gastric acid secretion. After oral administration, it is absorbed through the gastrointestinal tract quickly. Synthetic route [4]
The intermediates (3) and (5) were synthesized as follows. The intermediates (3) and (5) were then reacted to produce (6) ranitidine. The (6) is subject to purification and salt formation to generate ranitidine hydrochloride, as shown:
 | [Pharmacokinetics]
After oral administration, it is absorbed rapidly from the gastrointestinal tract with a bioavailability (F) of about 50% and peak plasma concentration (tmax) reaching within 1 to 2 hours. Plasma protein binding rate is 15% ± 3%; effective blood concentration is 100ng / ml, widely distributed in the body with the apparent volume of distribution (Vd) of 1.1 ~ 1.9L / Kg. It can penetrate through the blood brain barrier with the drug concentration in cerebrospinal fluid being 1/30 ~ 1 / 20 of blood concentration. 30% is subject to liver metabolism with the metabolites including N-oxide, S-oxide and demethylation metabolites. 50% of the prototype is subject to urine excretion through kidney. Half-life (t1 / 2) is 2 to 3 hours, which is similar to cimetidine. Renal failure causes corresponding increase in the half-life. The goods can be transplanted via the placenta with the milk concentration being higher than the plasma concentration of drugs.
| [Indications]
For the treatment of duodenal ulcer, gastric ulcer, reflux esophagitis, Zollinger-Ellison syndrome and other high acid secretion disorders.
| [Adverse reactions]
- Common reactions are: nausea, rash, constipation, fatigue, headache, dizziness and so on.
- Light adverse reactions on the renal function, gonadal function and central nervous system.
- A small number of patients get mild liver damage after taking the drug with the symptoms disappearing after stopping, liver function returned to normal.
| [Medicine interactions]
- Combination with procainamide reduces the clearance rate of the later one.
- Drug interactions may occur if used with other medications, consult physician or pharmacist for details.
| [Precautions]
- This product mustn’t be used continuously for more than 7 days, the symptom is not relieved, consult your physician or pharmacist.
- Elderly patients or patients with liver and kidney dysfunction use with caution.
- If overdose or serious adverse reactions, should seek medical treatment immediately.
- Disable it for people who are allergic to this product; allergies should use with caution.
- This product is not allowed for administration upon change of its traits.
- Please put this product where children cannot touch.
- Children must be under adult guardianship.
- If you are using other drugs, please consult your physician or pharmacist before using this product.
| [Contraindication]
- Children under 8 years should be disabled.
- Pregnant and lactating women should be disabled.
| [Elderly patients medication]
The elderly have reduced liver and kidney function; in order to ensure drug safety, dosage should be adjusted.
|
|
|