| Identification | More | [Name]
2,6-Dichlorophenylacetic acid | [CAS]
6575-24-2 | [Synonyms]
2-(2,6-DICHLOROPHENYL)ACETIC ACID
2,6-DICHLORO BENZENEACETIC ACID
2,6-DICHLOROPHENYLACETIC ACID
RARECHEM AL BO 0115
TIMTEC-BB SBB003503
Benzeneacetic acid, 2,6-dichloro-
2,6-Dichlorophenyl acetic acid 98%
2,6-Dichlorophenylacetic acid, 98+%
Acetic acid, (2,6-dichlorophenyl)- | [EINECS(EC#)]
229-504-0 | [Molecular Formula]
C8H6Cl2O2 | [MDL Number]
MFCD00004320 | [Molecular Weight]
205.04 | [MOL File]
6575-24-2.mol |
| Safety Data | Back Directory | [Hazard Codes ]
Xi | [Risk Statements ]
R36/37/38:Irritating to eyes, respiratory system and skin . | [Safety Statements ]
S37/39:Wear suitable gloves and eye/face protection .
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice .
S36:Wear suitable protective clothing . | [WGK Germany ]
3 | [Hazard Note ]
Irritant | [HS Code ]
29163900 |
| Hazard Information | Back Directory | [Chemical Properties]
white crystalline powder | [Uses]
2,6-Dichlorophenylacetic acid is an inhibitor of isopenicillin N synthase (IPNS) and acyl-CoA: 6-APA acyltransferase. 2,6-Dichlorophenylacetic acid is also part of a group of phenylacetate derivatives that have cytostatic activity against tumour cells. | [Preparation]
The preparation of the 2, 6-dichlorophenylacetic acid:
the method comprises the following steps: 2, 6-dichlorotoluene is used as a raw material, and is catalyzed by a complex catalyst formed by transition metal and a ligand (wherein, a transition metal catalyst precursor is preferably palladium chloride, an oxidant is preferably TBP (tert-butyl peroxy ether), and a ligand is preferably Xantphos (4, 5-bis (diphenylphosphino) -9, 9-dimethyl xanthene)) in the presence of an alcohol and a catalyst and an oxidant to obtain 2, 6-dichlorophenylacetic acid, and the ethyl 2, 6-dichlorophenylacetate is prepared by hydrolysis and acidification, wherein the total yield is 68.4%. See patent document US2013303798, the reaction procedure is described as synthetic route 1.
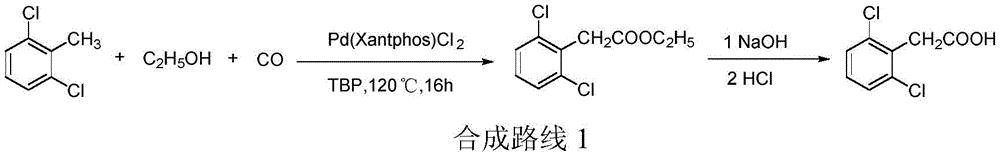
In the above synthetic route 1, the preparation process of the intermediate ethyl 2, 6-dichlorophenylacetate requires the use of carbon monoxide for high-temperature and high-pressure reaction, and has poor operation safety, high equipment requirement, high cost, and is not favorable for cost reduction and green production of 2, 6-dichlorophenylacetic acid. | [Purification Methods]
Crystallise the acid from aqueous EtOH. [Beilstein 9 III 2272.] |
|
|