| Identification | Back Directory | [Name]
Nicorandil | [CAS]
65141-46-0 | [Synonyms]
SG-75
Dancor
IKOREL
ADANCOR
SIGMART
Zynicor
PERISALOL
NICORANDIL
Nicorandil(Ikorel)
2-nicotinamidoethylnitrate
N-(2-Nitrooxyethyl)nicotinaMide
Adancor, Ikorel, Perisalol, Sigmart
2-(pyridin-3-ylforMaMido)ethyl nitrate
N-[2-Hydroxyethyl] nicotinamide nitrate
Nitric acid 2-nicotinoylaminoethyl ester
2-(pyridine-3-carbonylamino)ethyl nitrate
2-(pyridin-3-ylcarbonylamino)ethyl nitrate
n-(2-(nitrooxy)ethyl)-3-pyridinecarboxamid
n-[2-(nitroxy)ethyl]-3-pyridinecarboxamide
N-[2-(NITROOXY)ETHYL]-3-PYRIDINECARBOXAMIDE
n-(2-hydroxyethyl)nicotinamidenitrate(ester)
N-(2-Hydroxyethyl)nieotimmide nitrate(ester)
3-Pyridinecarboxamide, N-[2-(nitrooxy)ethyl]-
nitric acid 2-(pyridine-3-carbonylamino)ethyl ester
2-(Pyridine-3-carboxamido)ethyl Nitrate
N-[2-(Nitrooxy)ethyl]pyridine-3-carboxamide
2-[(Pyridin-3-ylcarbonyl)amino]ethyl nitrate, N-[2-(Nitroxy)ethyl]pyridine-3-carboxamide | [EINECS(EC#)]
265-514-1 | [Molecular Formula]
C8H9N3O4 | [MDL Number]
MFCD00186520 | [MOL File]
65141-46-0.mol | [Molecular Weight]
211.17 |
| Chemical Properties | Back Directory | [Appearance]
White solid | [Melting point ]
92°C | [Boiling point ]
350.85°C (rough estimate) | [density ]
1.4271 (rough estimate) | [refractive index ]
1.7400 (estimate) | [RTECS ]
US4667600 | [storage temp. ]
Desiccate at +4°C | [solubility ]
DMSO: >10mg/mL | [form ]
powder | [pka]
pKa 空 (Uncertain) | [color ]
white to off-white | [Merck ]
14,6521 | [CAS DataBase Reference]
65141-46-0 |
| Safety Data | Back Directory | [Hazard Codes ]
Xn | [Risk Statements ]
22-41 | [Safety Statements ]
26-39 | [WGK Germany ]
3 | [HS Code ]
29333990 | [Toxicity]
LD50 in rats (mg/kg): 1200-1300 orally; 800-1000 i.v. (Nagano) |
| Hazard Information | Back Directory | [Chemical Properties]
Nicorandil is N-(2-hydroxyethyl)-nicotinamide nitrate (ester). It is a white crystalline powder or white needles with a faint, characteristic odour. It is freely soluble in methanol, ethanol, acetone or glacial acetic acid, slightly soluble in chloroform or water, almost insoluble in ether or benzene. Melting point 88.5-93.5 ℃. Acute toxicity LD50 rat (mg/kg): 1200-1300 orally, 800-1000 intravenously. | [Originator]
Chugai (Japan) | [Uses]
Nicorandil is a vasodilator used for the prophylaxis and treatment of angina. This medication widens blood vessels thus increasing the blood and oxygen flow to the heart. It is not currently available in the US.
Nicorandil is a nitric oxide donor and ATP-sensitive potassium channel opener. A study showed that Nicorandil is able to protect against stress-induced cell death in dystrophin-deficient cardiomyocytes and also preserve cardiac function in the mdx mouse heart subjected to ischemia and reperfusion injury. | [Definition]
ChEBI: Nicorandil is a pyrimidinecarboxamide that is nicotinamide in which one of the hydrogens attached to the carboxamide nitrogen is replaced by a 2-(nitrooxy)ethyl group. It has both nitrate-like and ATP-sensitive potassium channel activator properties, and is used for the prevention and treatment of angina pectoris. It has a role as a vasodilator agent and a potassium channel opener. It is a pyridinecarboxamide and a nitrate ester. It derives from a nicotinamide. | [Preparation]
Nicorandil is obtained by treating nicotinic acid chloride hydrochloride with nitro-oxy-ethyl-amine nitrate in an organic solvent.
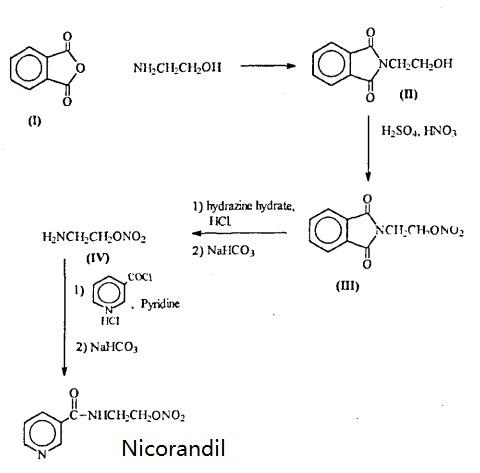
Nicorandil was synthesized from 2-aminoethanol by a four step process. In order to protect the amino group during nitration, 2-aminoethanol was first treated with phthalic anhydride. The amide formed was then nitrated using a nitrating mixture and was subsequently deprotected by reacting with hydrazine hydrate. The 2- nitroxyethylamine obtained was condensed with nicotinoyl chloride in pyridine. Treatment with aqueous sodium bicarbonate solution gave nicorandil as free base.
synthesis of Nicorandil | [Brand name]
SIGMART; PERISALOL | [Biochem/physiol Actions]
Nicorandil is a hybrid ATP-sensitive K+ (KATP) channel opener and nicotinamide nitrate NO donor. Nicorandil selectively activates SUR2B- versus SUR2A-containing KATP channels. It enhances endothelial NO synthase expression and protects against ischemic ventricular arrhythmias. By activating potassium channels, and donating nitric oxide to activate the enzyme guanylate cyclase, Nicorandil causes activation of GMP leading to both arterial and venous vasodilatation. Nicorandil is selective for vascular potassium channels, but has no significant action on cardiac contractility and conduction. | [Clinical Use]
Nicorandil is used for the prevention and treatment of chronic stable angina. It is usually used when angina becomes difficult to control by other drugs and is given as an additional treatment. | [Drug interactions]
Potentially hazardous interactions with other drugs
Avanafil, sildenafil, tadalafil and vardenafil: enhanced
hypotensive effect - avoid.
Riociguat: possible enhanced hypotensive effect -
avoid. | [Metabolism]
Metabolism of nicorandil is mainly by denitration of the
molecule into the nicotinamide pathway.
About 20% of a dose is excreted in the urine mainly as
metabolites | [storage]
Store at -20°C | [Mode of action]
The coronary vasodilating activity of nicorandil is considered to be the consequence of the increasing cyclic GMP production by stimulating guanylyl cyclase in the coronary vascular smooth muscle, similar to other nitrates/nitrites (in vitro). In addition, other mechanisms such as hyperpolarization of the cell membrane were investigated concerning its coronary blood flowincreasing and coronary vasospasmolytic effects.
The vasorelaxant effect of nicorandil in isolated blood vessels is suppressed by an ATP-sensitive K channel blocker or a guanylate cyclase inhibitor. Moreover, in a canine model of acute heart failure, the cardiac haemodynamics-improving effect of the drug (e.g. effect of increasing the aortic blood flow) was suppressed by an ATP-sensitive K channel blocker. Furthermore, the drug caused an increase in the cGMP content in isolated blood vessels. These facts suggest that the vasodilating effect of the drug is related to the effect of opening the ATP-sensitive K channel, as well as to the effect of increasing production of cGMP. |
|
|