| Identification | More | [Name]
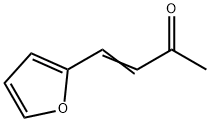
4-(2-FURYL)-3-BUTEN-2-ONE | [CAS]
623-15-4 | [Synonyms]
[2-(2-FURYL)-VINYL]-METHYLKETON
4-(2-FURYL)-3-BUTEN-2-ONE
4-(2-FURYL)BUT-3-EN-2-ONE
4-FURYL-3-BUTEN-2-ONE
B-2-FURYLIDENEACETONE
(E)-4-(FURAN-2-YL)BUT-3-EN-2-ONE
FEMA 2495
FURFURALACETONE
FURFURYLIDENEACETONE
LABOTEST-BB LT00039163
(3E)-4-(2-Furyl)-3-buten-2-one
1-(2-Furyl)but-2-en-3-one
2-furfurylideneacetone
3-Buten-2-one, 4-(2-furyl)-
3-Butene-2-one, 4-(2-furanyl)-
4-(2-furanyl)-3-buten-2-on
4-(2-furanyl)-3-buten-2-one
4-(2-furyl)-3-buten-2-on
beta-2-Furylideneacetone
FAM (monomer) | [EINECS(EC#)]
210-774-3 | [Molecular Formula]
C8H8O2 | [MDL Number]
MFCD00039566 | [Molecular Weight]
136.15 | [MOL File]
623-15-4.mol |
| Chemical Properties | Back Directory | [Appearance]
reddish crystalline powder | [Melting point ]
34-41 °C(lit.)
| [Boiling point ]
112-115°C 10mm | [density ]
1.0496 | [FEMA ]
2495 | [refractive index ]
n20/D 1.565(lit.)
| [Fp ]
220 °F
| [storage temp. ]
Keep in dark place,Inert atmosphere,2-8°C | [form ]
powder to lump | [color ]
Light yellow to Brown | [Odor]
at 1.00 % in propylene glycol. sweet spicy warm balsam cinnamon vanilla | [Stability:]
Flammable. Incompatible with strong oxidizing agents. | [Odor Type]
spicy | [Sensitive ]
Light Sensitive | [JECFA Number]
1511 | [BRN ]
109696 | [LogP]
1.79 | [CAS DataBase Reference]
623-15-4(CAS DataBase Reference) | [EPA Substance Registry System]
Furfural acetone (623-15-4) |
| Safety Data | Back Directory | [Hazard Codes ]
Xi | [Risk Statements ]
R36/37/38:Irritating to eyes, respiratory system and skin . | [Safety Statements ]
S37/39:Wear suitable gloves and eye/face protection .
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice . | [WGK Germany ]
3
| [RTECS ]
EM9939000
| [Hazard Note ]
Irritant | [TSCA ]
Yes | [HS Code ]
29321900 |
| Hazard Information | Back Directory | [General Description]
Reddish crystalline solid. | [Reactivity Profile]
An aldehyde and a ketone. Aldehydes are frequently involved in self-condensation or polymerization reactions. These reactions are exothermic; they are often catalyzed by acid. Aldehydes are readily oxidized to give carboxylic acids. Flammable and/or toxic gases are generated by the combination of aldehydes with azo, diazo compounds, dithiocarbamates, nitrides, and strong reducing agents. Aldehydes can react with air to give first peroxo acids, and ultimately carboxylic acids. These autoxidation reactions are activated by light, catalyzed by salts of transition metals, and are autocatalytic (catalyzed by the products of the reaction). Ketones are reactive with many acids and bases liberating heat and flammable gases (e.g., H2). The amount of heat may be sufficient to start a fire in the unreacted portion of the ketone. Ketones react with reducing agents such as hydrides, alkali metals, and nitrides to produce flammable gas (H2) and heat. Ketones are incompatible with isocyanates, aldehydes, cyanides, peroxides, and anhydrides. They react violently with aldehydes, HNO3, HNO3 + H2O2, and HClO4. | [Air & Water Reactions]
Slightly water soluble. | [Fire Hazard]
This chemical is flammable. | [Chemical Properties]
4-(2-Furyl)-3-buten-2-one has a sweet spicy odor and taste It is useful in nut favors. | [Chemical Properties]
reddish crystalline powder | [Occurrence]
Reported found in coffee and rum | [Uses]
4-(2-Furyl)-3-buten-2-one, can be used in technical or engineered material industyr. It has shown to have the durability need in composites. | [Taste threshold values]
Taste characteristics at 20 ppm: sweet, nutty, powdery, vanilla and coumarin creamy. | [Synthesis Reference(s)]
Journal of the American Chemical Society, 106, p. 6735, 1984 DOI: 10.1021/ja00334a044
The Journal of Organic Chemistry, 52, p. 4855, 1987 DOI: 10.1021/jo00231a006 |
|
|