| Identification | Back Directory | [Name]
(S)-1-(3-Ethoxy-4-Methoxyphenyl)-2-(Methylsulfonyl)ethylaMine N-acetyl-L-leucine salt | [CAS]
608141-43-1 | [Synonyms]
Apremilast Impurity 62
(2S)-2-acetamido-4-methylpentanoate
Apremilast Impurity 55(N-Acetyl-L-leucine Salt)
(S)-1-(3-Ethoxy-4-methoxyphenyl)-2-(methylsulfonyl)ethylamin...
[(1S)-1-(3-ethoxy-4-methoxyphenyl)-2-methylsulfonylethyl]azanium
(S)-1-(3-ethoxy-4-methoxyphenyl)-2-(methylsulfonyl)ethan-1-amine acetyl-L-leucinate
(S)-1-(3-Ethoxy-4-methoxyphenyl)-2-(methylsulfonyl)ethanamine N-Acetyl-L-leucine Salt
(S)-1-(3-Ethoxy-4-Methoxyphenyl)-2-(Methylsulfonyl)ethylaMine N-acetyl-L-leucine salt
(1S)-1-(3-ethoxy-4-methoxyphenyl)-2-(methanesulfonyl)ethylamine N-acetyl L-leucine salt
(S)-1-(3-Ethoxy-4-methoxyphenyl)-2-(methylsulfonyl)ethanamine (S)-2-acetamido-4-methylpentanoate
L-Leucine, N-acetyl-, compd. with(aS)-3-ethoxy-4-methoxy-a-[(methylsulfonyl)methyl]benzenemethanamine (1:1)
N-Acetyl-L-leucine compound with (alphaS)-3-ethoxy-4-methoxy-alpha-[(methylsulfonyl)methyl]benzenemethanamine | [Molecular Formula]
C12H19NO4S.C8H15NO3 | [MDL Number]
MFCD28167941 | [MOL File]
608141-43-1.mol | [Molecular Weight]
446.56 |
| Hazard Information | Back Directory | [Synthesis]
(S)-1-(3-Ethoxy-4-Methoxyphenyl)-2-(Methylsulfonyl)ethylaMine N-acetyl-L-leucine salt is prepared by the reaction of 1-(3-ethoxy-4-methoxyphenyl)-2-(methylsulfonyl)ethanamine and N-Acetyl-L-leucine. The specific synthesis steps are as follows:
A 3 L 3-necked round bottom flask was equipped with a mechanical stirrer, thermometer, and condenser and charged with 2-(3 -ethoxy-4-methoxyphenyl)- 1 -(methylsulphonyl)-eth-2-ylamine (137.0 g, 500 mmol), N-acetyl-L-leucine (52 g, 300 mmol), and methanol (1.0 L). The stirred slurry was heated to reflux for 1 hour. The stirred mixture was allowed to cool to ambient temperature and stirring was continued for another 3 hours at ambient temperature. The slurry was filtered andwashed with methanol (250 mL). The solid was air-dried and then dried in vacuo at ambient temperature to a constant weight, giving 109.5 g (98percent yield) of the crude product (85.8percent ee). The crude solid (55.0 g) and methanol (440 mL) were brought to reflux for 1 hour, cooled to room temperature and stirred for an additional 3 hours at ambient temperature. The slurry was filtered and the filter cake was washed with methanol (200 mL). The solid was air-dried and then dried invacuo at 30°C. to a constant weight, yielding 49.6 g (90percent recovery) of(S)-2-(3-ethoxy-4-methoxyphenyl)- 1 -(methylsulphonyl)-eth-2-ylamine-N-acety l-L-leucine salt (98.4percent ee). ChiralHPLC (1/99 EtOH/20 mM KH2PO4 pH 7.0, Ultron Chiral ES-OVS from Agilent Technologies,150 mm4.6 mm, 0.5 mL/min., 240 nm): 18.4 mm (S-isomer, 99.2percent), 25.5 mm (R-isomer,0.8percent).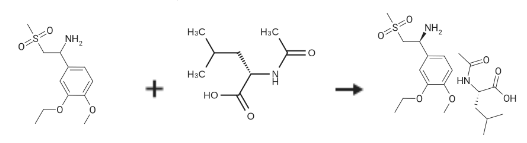
|
|
|