| Identification | More | [Name]
4-Chloro-2,3-dimethylpyridine 1-oxide | [CAS]
59886-90-7 | [Synonyms]
2,3-DIMETHYL-4-CHLOROPYRIDINE-N-OXIDE
4-CHLORO-2,3-DIMETHYL-PYRIDINE 1-OXIDE
4-CHLORO-2,3-DIMETHYLPYRIDINE-N-OXIDE
nsc 275262
2,3-Dimethyl-4-Chloro Pyridine-1-Oxide
NSC 27526
Pyridine, 4-chloro-2,3-dimethyl-, 1-oxide
4-Chloro-2,3-Dimethyl Pyridine-N-Oxide 99%
Pyridine-N-oxide, 4-chloro-2,3-dimethyl
4-Chloro-2,3-dimethylpyridine-N-oxide ,98% | [EINECS(EC#)]
611-910-8 | [Molecular Formula]
C7H8ClNO | [MDL Number]
MFCD06411125 | [Molecular Weight]
157.6 | [MOL File]
59886-90-7.mol |
| Chemical Properties | Back Directory | [Appearance]
Pale Yellow Solid | [Melting point ]
103-105°C | [Boiling point ]
338.5°C | [density ]
1.19 | [vapor pressure ]
0.346Pa at 25℃ | [Fp ]
158.5°C | [storage temp. ]
Room temperature. | [solubility ]
Chloroform (Slightly), Methanol (Slightly) | [form ]
Solid | [pka]
1.28±0.10(Predicted) | [color ]
Pale Yellow to Light Beige | [Detection Methods]
HPLC,NMR | [LogP]
0.4 at 25℃ | [CAS DataBase Reference]
59886-90-7(CAS DataBase Reference) |
| Hazard Information | Back Directory | [Chemical Properties]
Pale Yellow Solid | [Uses]
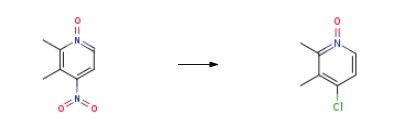
An intermediate in the synthesis of rabeprazole (R070500). | [Synthesis]
83.0 g (0.49 mol) of 4-nitro-2,3-dimethylpyridine N-oxide and 90 g (1.54 mol) of NaCl were admixed with 1350 ml of CH3CN, 180 ml of aq. HCl (36%) and 16.6 g of benzyltributylammonium chloride, and the resulting suspension was boiled under reflux with stirring for 12 h. The resulting reaction mixture was adjusted to PH=9 using 350 ml of 20% NaOH, wherefor the existing precipitate largely dissolved. The organic phase was separated off, water was added to the aqueous phase until the precipitate had completely dissolved, and it was subsequently extracted using dichloromethane. The organic phases were combined and the solvent was removed under reduced pressure. 76.6 g (98.5%) of 4-Chloro-2,3-dimethylpyridine 1-oxide were obtained.
 |
|
|