| Identification | More | [Name]
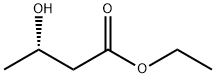
Ethyl (S)-3-hydroxybutyrate | [CAS]
56816-01-4 | [Synonyms]
ETHYL-[(3S)-(+)-3-HYDROXYBUTYRAT]
ETHYL (3S)-3-HYDROXYBUTYRATE
ETHYL S-(+)-3-HYDROXYBUTANOATE
ETHYL (S)-(+)-3-HYDROXYBUTYRATE
ETHYL (S)-3-HYDROXYBUTYRATE
(S)-(+)-3-HYDROXYBUTYRIC ACID ETHYL ESTER
(S)-3-HYDROXYBUTYRIC ACID ETHYL ESTER
(S)-(+)-3-HYDROXY-N-BUTYRIC ACID ETHYL ESTER
(S)-(+)-ETHYL 3-HYDROXYBUTYRATE
(R)-2-HEPTANOL EE 97+%
Butanoic acid, 3-hydroxy-, ethyl ester, (3S)-
(S)-ETHYL-3-HYDROXYBUTYRATE 99+%
(3S)-3-Hydroxybutanoic acid ethyl
(3S)-3-Hydroxybutyric acid ethyl
(S)-3-Hydroxybutanoic acid ethyl
(S)-3-Hydroxybutyric acid ethyl | [EINECS(EC#)]
260-393-1 | [Molecular Formula]
C6H12O3 | [MDL Number]
MFCD00066206 | [Molecular Weight]
132.16 | [MOL File]
56816-01-4.mol |
| Chemical Properties | Back Directory | [Appearance]
clear colorless liquid | [alpha ]
43 º (C=1, CHLOROFORM) | [Boiling point ]
180-182 °C (lit.) | [density ]
1.012 g/mL at 20 °C(lit.)
| [refractive index ]
n20/D 1.421(lit.)
| [Fp ]
148 °F
| [storage temp. ]
Sealed in dry,Room Temperature | [solubility ]
Soluble in chloroform | [form ]
Liquid | [pka]
14.45±0.20(Predicted) | [color ]
Colorless to Yellow | [optical activity]
[α]20/D +43°, c = 1 in chloroform | [BRN ]
2347951 | [LogP]
-0.019 (est) | [CAS DataBase Reference]
56816-01-4(CAS DataBase Reference) |
| Safety Data | Back Directory | [Safety Statements ]
S23:Do not breathe gas/fumes/vapor/spray (appropriate wording to be specified by the manufacturer) .
S24/25:Avoid contact with skin and eyes . | [WGK Germany ]
3
| [HS Code ]
29181990 |
| Hazard Information | Back Directory | [Description]
Ethyl (S)-3-hydroxybutyrate is a chiral building block for the preparation of bioactive compounds such as pheromones and carbapenem antibiotics. | [Chemical Properties]
clear colorless liquid | [Uses]
Ethyl (S)-(+)-3-hydroxybutyrate is used for synthesis of optically active products, used as medical and organic intermediates, an important amino protective agents, pharmaceuticals and synthetic organic makes intermediates, organic synthesis used to introduce t-Boc protect gene. | [Application]
Ethyl (S)-3-hydroxybutyrate may be used in the preparation of 3-(1′-hydroxyethyl)-2-azetidinones. | [General Description]
Ethyl (S)-3-hydroxybutyrate is a chiral building block for the preparation of bioactive compounds such as pheromones and carbapenem antibiotics. |
|
|