| Identification | More | [Name]
Demecarium bromide | [CAS]
56-94-0 | [Synonyms]
3,3'-(1,10-decanediylbis((methylimino)carbonyloxy))bis(n,n,n-trimethyl-benzenaminium)) dibromide
demecarium bromide
(m-hydroxyphenyl)trimethylammoniumbromidedecamethylenebis(methylcarbamate)
2br
ammonium,(m-hydroxyphenyl)trimethyl-,bromide,decamethylenebis(methylcarbamat
bc48
benzenaminium,3,3’-(1,10-decanediylbis((methylimino)carbonyloxy))bis(n,n,n-t
benzenaminium,3,3’-(1,10-decanediylbis((methylimino)carbonyloxy))bis(n,n,n-tri
decamethylenebis(m-dimethylaminophenyl-n-methylcarbamate)dimethobromide
decamethylenebis(n-methylcarbamicacidm-dimethylaminophenylester)bromomethyl
demekariumbromide
demekastigminebromide
e)(2:1)
frumtosnil
humorsol
n,n’-bis(3-trimethylammoniumphenoxycarbonyl)-n,n’-dimethyldecamethylenediamine
tonilen
tosmicil
tosmilen
tosmilene | [EINECS(EC#)]
200-301-9 | [Molecular Formula]
C32H52Br2N4O4 | [MDL Number]
MFCD00152376 | [Molecular Weight]
716.59 | [MOL File]
56-94-0.mol |
| Chemical Properties | Back Directory | [Melting point ]
162-167 °C (decomp) | [storage temp. ]
Store at -20°C | [solubility ]
DMSO:160.0(Max Conc. mg/mL);223.28(Max Conc. mM) | [form ]
Solid | [color ]
White | [InChIKey]
YHKBUDZECQDYBR-UHFFFAOYSA-L | [SMILES]
C1(=CC=CC([N+](C)(C)C)=C1)OC(=O)N(C)CCCCCCCCCCN(C)C(=O)OC1=CC=CC([N+](C)(C)C)=C1.[Br-].[Br-] | [CAS DataBase Reference]
56-94-0(CAS DataBase Reference) |
| Hazard Information | Back Directory | [Chemical Properties]
White Solid | [Originator]
Humorsol ,MSD ,US,1959 | [Uses]
anticancer agent for pancreatic islet cells | [Uses]
Demecarium Bromide is an acetylcholinesterase inhibitor and veterinary medicine used to treat elevated intraocular pressure associated with primary glaucoma. | [Definition]
ChEBI: The methobromide salt of the N,N'-bis[3-(dimethylamino)phenyl carbamate] derivative of 2,13-diazatetradecane. It is an inhibitor of acetylcholinesterase and pseudocholinesterase, with a long duration of action. It is u
ed in the treatment of chronic open-angle glaucoma: in the eye, it causes constriction of the iris sphincter muscle and the ciliary muscle, facilitating the outflow of the aqueous humor and so reducing intraocular pressure. | [Manufacturing Process]
N,N,N,N'-tetramethyldecamethylene diamine is reacted with phosgene in
toluene under agitation. The phosgene which escapes through an ascending
cooling tube together with the evolved methyl chloride is condensed in a cold
trap. As soon as immixture has been completed, the temperature is raised to
100°C and the phosgene recovered in the trap is vaporized and bubbled
through the solution again, the escaping gas being recondensed and returned
once more. The repeated passage through the reagents of the phosgene that
has not yet reacted is continued for 7 hours. When the solution is cool it is
passed through a filter, the remaining phosgene is removed from the clear
solution by distillation and the remainder distilled in vacuo.
A solution of 11.9 parts of m-dimethylaminophenol in 90 parts of xylene
(isomer mixture) is added to a solution of sodium methylate consisting of 2.0
parts of sodium and 25 parts of methanol. The methanol is then completely
removed by distillation and the temperature raised until the boiling point of
the xylene is reached. The decamethylene-bis-(N-methyl carbamic chloride) is
added to the remainder which contains the sodium salt of m dimethylaminophenol in the form of solid crystals. The reagent mixture is
heated and maintained at a temperature of 100°C and continuously agitated.
After having been cooled it is washed three times in water, three times in a
5% solution of caustic soda, and another three times in water. The xylene is
then evaporated in vacuo and the oily residue freed of any remaining traces of
xylene by allowing it to stand in air when the product crystallized completely.
In this manner 15.6 parts of decamethylene-bis-(N-methyl carbamic acid mdimethylaminophenylester) are obtained, This is in turn reacted with methyl
bromide to give the desired product. The decamethylene-bis-(N-methyl
carbamic acid m-dimethylaminophenylesterbromomethylate) appears after
precipitation from a solution in acetic acid with methyl ethyl ketone in the
form of a finely crystalline powder with a micro melting point between 164°
and 170°C. | [Brand name]
Humorsol (Merck). | [Therapeutic Function]
Cholinergic (ophthalmic) | [General Description]
Demecarium bromide,(m-hydroxyphenyl)trimethylammonium bromide, decamethylenebis[methylcarbamate] (Humorsol), is the diester of(m-hydroxyphenyl)trimethylammonium bromide with decamethylene-bis-(methylcarbamic acid) and thus is comparableto a bis-prostigmine molecule.
It occurs as a slightly hygroscopic powder that is freelysoluble in water or alcohol. Ophthalmic solutions of the drughave a pH of 5 to 7.5. Aqueous solutions are stable and maybe sterilized by heat. Its efficacy and toxicity are comparableto those of other potent anticholinesterase inhibitor drugs. Itis a long-acting miotic used to treat wide-angle glaucomaand accommodative esotropia. Maximal effect occurs hoursafter administration, and the effect may persist for days. | [Synthesis]
Demecarium, N,N??-decamethylene-bis-[meta-(N-methylcarbamoyloxy)-
phenyl-trimethylammonium] hydroxide (13.2.18), is made by reacting two moles of phos�gene with 1,10-bis-(methylamino)-decano(N,N??-dimethyldecamethylen-1,10-diamine,
giving the bis-carbamoylchloride (13.2.16), which is transformed into bis-carbamoylester
(13.2.17) by reaction with two moles of the 3-dimethylaminophenol sodium salt. Reacting
this with methylbromide gives demecarium (13.2.18) [49].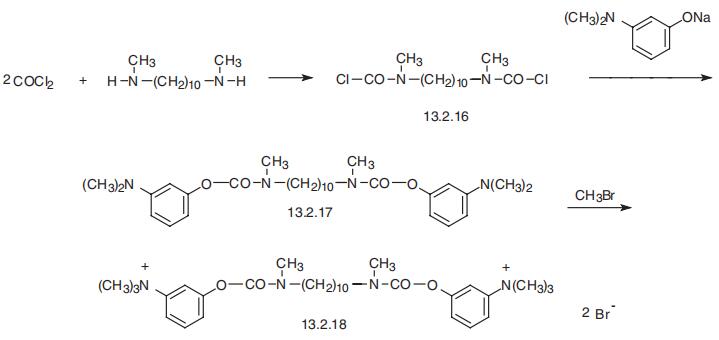
| [Veterinary Drugs and Treatments]
Demecarium is a potent carbamate inhibitor that may reduce intraocular
pressures for up to 48 hours in canines. Demecarium
reversibly inhibits anticholinesterase thereby causing miosis.
Demecarium is generally used in preventive management of the
contralateral eye in canine patients after the diagnosis of an acute
congestive crisis of primary glaucoma in the other eye. It is not used
in secondary glaucoma. Demecarium has the advantage of once or
twice daily dosing. |
|
|