| Identification | Back Directory | [Name]
Tris(dibenzylideneacetone)dipalladium-chloroform adduct | [CAS]
52522-40-4 | [Synonyms]
Tris DBA
Pd2dba3.CHCl3
Tris DBA chloroform adduct
52522-40-4
tris(dibenzylideneacetone)(chloroform)-di-p
Tris(dibenylideneacetone)dipalladium-chloroform
TRIS(DIBENZYLIDENEACETONE)DIPALLADIUM-CHLOROFORM
Tris(dibenzylideneacetone)dipalladium(0)-chloroform
TRIS(DIBENZYLIDENEACETONE)(CHLOROFORM)-DI-PALLADIUM(0)
TRIS(DIBENZYLIDENEACETONE)DIPALLADIUM-CHLOROFORM ADDUCT
Dipalladiumtris(dibenzylideneacetone) chloroform adduct
TRIS(DIBENZYLIDENEACETONE)DIPALLADIUM CHLOROFORM COMPLEX
Tris(Dibenzylideneacetone)DipalladiuM- ChloroforM AdduCL
DI-PALLADIUM-TRIS(DIBENZYLIDENEACETONE)CHLOROFORM COMPLEX
DIPALLADIUM(0)TRIS(DIBENZYLIDENEACETONE)-CHLOROFORM ADDUCT
TRIS(DIBENZYLIDENEACETONE)DIPALLADIUM(0) CHLOROFORM ADDUCT
Tris(dibenzylideneacetone)dipalladium-chloroform adduct,97%
Tris-(dibenzylideneacetone)-dipalladium(O)-chloroform adduct
Tris (dibenzylidene acetone)di palladiuM(O)-chloroforM addict
chloroform,(1E,4E)-1,5-diphenylpenta-1,4-dien-3-one,palladium
TRIS(DIBENZYLIDENEACETONE)DIPALLADIUM(0) CHLOROFORM ADDUCT 98+%
Tris(dibenzylideneacetone)dipalladiuM-chloroforM adduct, 97% 1GR
Tris(Dibenzylideneacetone)Dipalladium(0), Complex With Chloroform, Pd
Tris(dibenzylideneacetone)dipalladium(0) chloroform adduct [Pd > 20.2%]
Tris(dibenzylideneacetone)dipalladium(0), complex with chloroform, Pd 20.6%
Tris(dibenzylideneacetone)dipalladiuM (0) chloroforM adduct, Pd2(dba)3·CHCl3
2,2'',3',6,6'',7'-hexaphenyldispiro[1-palladatricyclo[3.1.0.0^{1,3}]hexane-1,2'-[1,2]dipalladatricyclo[4.1.0.0^{2,4}]heptane-1',1''-[1]palladatricyclo[3.1.0.0^{1,3}]hexane]-2,2'',3',5,5'',6'-hexaene-4,4'',5'-trione | [EINECS(EC#)]
-0 | [Molecular Formula]
C52H43Cl3O3Pd2 | [MDL Number]
MFCD00075479 | [MOL File]
52522-40-4.mol | [Molecular Weight]
1035.09 |
| Chemical Properties | Back Directory | [Appearance]
purple to black crystalline powder | [Melting point ]
131-135 °C(lit.)
| [storage temp. ]
0-6°C | [form ]
Crystalline Powder | [color ]
Purple to black | [Water Solubility ]
Soluble in organic solvents. Insoluble in water. | [Sensitive ]
Air & Moisture Sensitive | [InChIKey]
NLYJPAJWGANOKG-CXKPDERJSA-N |
| Hazard Information | Back Directory | [Chemical Properties]
purple to black crystalline powder | [Uses]
suzuki reaction | [Biological Activity]
N -myristoyltransferase-1 (NMT-1) inhibitor that prevents activation of MAPK, PI 3-K and STAT3 signaling pathways. Exhibits antiproliferative activity against melanoma cells in vitro and in vivo . Also used as a cyclization catalyst. | [Application]
Tris(dibenzylideneacetone)dipalladium-chloroform adduct (Pd2dba3·CHCl3) was used in the following studies:
To compose the catalytic system for the preparation of homoallylpalladium complexes.These complexes underwent in situ Stille type cross coupling with various vinyltin reagents to afford the cyclized products bearing allyl appendages.
As palladium source in the asymmetric transformations of 3,4-epoxy-1-butene.
As catalyst for the Heck cross-coupling reaction of iodobenzene with styrene.
As cyclization catalyst.
As catalyst for [2+2+2] cycloaddtion of didehydrotriphenylenes to the corresponding extended triphenylenes.
As catalyst for the carbonylation of b,b-imidoyl iodides to the corresponding imidate esters used, in turn, to prepare cyclic, quaternary amino acids.
| [General Description]
Tris(dibenzylideneacetone)dipalladium(0)-chloroform adduct (Pd2dba3·CHCl3) is reported to serve as effective precatalysts, or precursors to the active Pd(0) catalyst. | [reaction suitability]
core: palladium
reaction type: Buchwald-Hartwig Cross Coupling Reaction
reaction type: Cross Couplings
reaction type: Heck Reaction
reaction type: Hiyama Coupling
reaction type: Negishi Coupling
reaction type: Sonogashira Coupling
reaction type: Stille Coupling
reaction type: Suzuki-Miyaura Coupling
reagent type: catalyst |
| Questions And Answer | Back Directory | [Reaction]
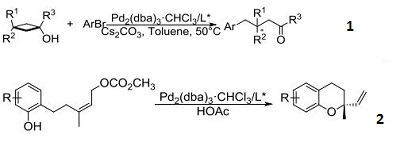
- Used for Pd-catalyzed asymmetric arylation, vinylation, and Allenylation of tert-cyclobutanols via enantioselective C−C Bond cleavage.
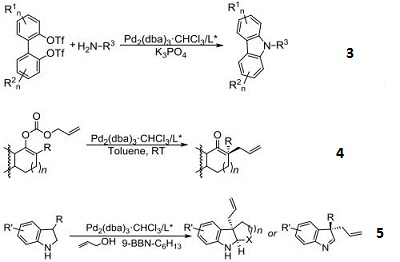
- Used for synthesis of chiral chromans through the Pd-catalyzed asymmetric allylic alkylation (AAA).
- Catalyst for double N-arylation of primary amines to synthesize multisubstituted carbazoles from 2,2‘biphenylylene ditriflates.
- Paladium catalyst for regioand enantioselective allylic alkylation of ketones through allyl enol carbonates.
- Used for Pd-catalyzed enantioselective C-3 allylation of 3-substituted-1H-indoles using trialkylboranes.
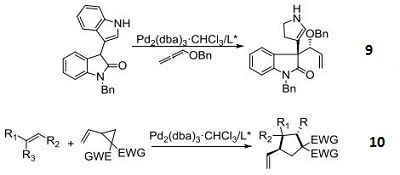
- Used for enantioselective construction of spirocyclic oxindolic cyclopentanes by Pd-catalyzed trimethylenemethane-[3+2]-cycloaddition.
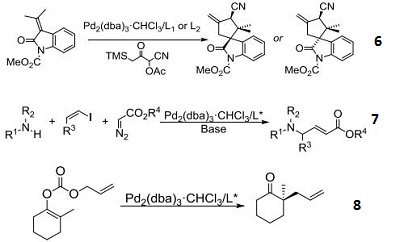
- Used for Pd-catalyzed insertion of α-diazoesters into vinyl halides to generate α,β-unsaturated γ-amino Esters.
- Pd catalyst for decarboxylative asymmetric allylic alkylation of enol carbonates.
- Palladium catalyst for asymmetric addition of oxindoles and allenes.
- Catalyst for diastereoand enantioselective formal [3+2]-cycloaddition between substituted vinylcyclopropanes and electron-deficient olefins.
- Used for Pd-catalyzed asymmetric decarboxylative cycloaddition of vinylethylene carbonates with Michael sacceptors.
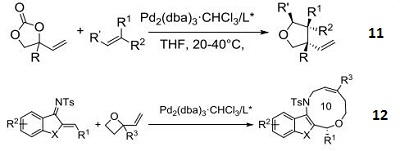
- Catalyst for enantioselective [6+4] cycloaddition of vinyl oxetanes with azadienes to access ten-membered heterocycles.
|
|
|