| Identification | More | [Name]
2-Iodopyridine | [CAS]
5029-67-4 | [Synonyms]
2-IODOPYRIDINE
2-Iodopyridine(stabilizedwithCopperchip)
2-lodopyridine
2-IODOPYRIDINE, 95+%
2-Pyridyl iodide | [Molecular Formula]
C5H4IN | [MDL Number]
MFCD00464928 | [Molecular Weight]
205 | [MOL File]
5029-67-4.mol |
| Chemical Properties | Back Directory | [Appearance]
Light yellow liquid | [Melting point ]
118-120℃ | [Boiling point ]
52 °C (lit.) | [density ]
1.928 g/mL at 25 °C(lit.)
| [refractive index ]
n20/D 1.6320(lit.)
| [storage temp. ]
Keep in dark place,Sealed in dry,Room Temperature | [form ]
Solid | [pka]
pK1:1.82(+1) (25°C) | [color ]
Pale yellow | [Water Solubility ]
Slightly soluble in water. | [Sensitive ]
Light Sensitive | [InChIKey]
CCZWSTFVHJPCEM-UHFFFAOYSA-N | [CAS DataBase Reference]
5029-67-4(CAS DataBase Reference) |
| Safety Data | Back Directory | [Hazard Codes ]
Xi | [Risk Statements ]
R36/37/38:Irritating to eyes, respiratory system and skin . | [Safety Statements ]
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice .
S36:Wear suitable protective clothing . | [WGK Germany ]
3
| [Hazard Note ]
Irritant | [HS Code ]
29333990 |
| Hazard Information | Back Directory | [Chemical Properties]
Light yellow liquid | [Uses]
2-Iodopyridine can be synthesized from 2-chloropyridine or 2-bromopyridine via treatment with iodotrimethylsilane.
2-Iodopyridine is a halogenated building block. It is a reagent used in the preparation of human NAD+-dependent 15-hydroxyprostaglandin dehydrogenase inhibitors. | [Reactions]
2-Iodopyridine and 3-alkoxy-2-iodopyridines are oxidized by DMDO to give PyIO2 , which serve as oxidants for alcohols and sulfides.
In palladium-catalyzed aminocarbonylation of 2-iodopyridine, 3-iodopyridine and iodopyrazine were coupled with CO and various primary and secondary amines. The biologically relevant N-substituted nicotinamides and 3-pyridyl-glyoxylamides were obtained from 3-iodopyridine as a result of simple and double carbon monoxide insertions, respectively. The chemoselectivity towards the ketoamide can be increased by the elevation of CO pressure. On the other hand, N-alkyl and N-aryl-carboxamides were obtained exclusively from CO pressure of 1 to 90 bar by using 2-iodopyridine and iodopyrazine.
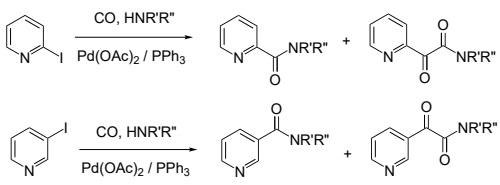
Pd-catalyzed aminocarbonylation of 2-iodopyridine and 3-iodopyridine with primary and secondary amines. | [Synthesis Reference(s)]
Tetrahedron Letters, 31, p. 6757, 1990 DOI: 10.1016/S0040-4039(00)97163-6 | [General Description]
2-Iodopyridine can be synthesized from 2-chloropyridine or 2-bromopyridine via treatment with iodotrimethylsilane. |
|
|