| Identification | Back Directory | [Name]
Eltrombopag | [CAS]
496775-61-2 | [Synonyms]
CS-640
Promacta
Sb497115
Sb-497115
ltrombopag
Eltrombopag
Unii-S56D65xj9g
Eltrombopag Acid
Eltrombopag [inn]
Eltrombopag Free Base
Eltrombopag (10mM in DMSO)
EltroMbopag (SB-497115-GR)
(E)-3'-(2-(1-(3,4-Dimethylphenyl)-3-methyl-5-oxo-1H-pyrazol-4(5H)-ylidene)hydrazinyl)-2'-hydro
(Z)-3'-(2-(1-(3,4-Dimethylphenyl)-3-methyl-5-oxo-1H-pyrazol-4(5H)-ylidene)hydrazinyl)-2'-hydro
(Z)-3'-(2-(1-(3,4-Dimethylphenyl)-3-methyl-5-oxo-1H-pyrazol-4(5H)-ylidene)hydrazinyl)-2'-hydroxy-
(E)-3\'-(2-(1-(3,4-Dimethylphenyl)-3-methyl-5-oxo-1H-pyrazol-4(5H)-ylidene)hydrazinyl)-2\'-hydroxybiphenyl-3-carboxylic acid
(Z)-3'-(2-(1-(3,4-DiMethylphenyl)-3-Methyl-5-oxo-1H-pyrazol-4(5H)-ylidene)hydrazinyl)-2'-hydroxy-[1,1'-biphenyl]-3-carboxylic acid
3-(5-{2-[2-(3,4-diMethylphenyl)-5-Methyl-3-oxo-2,3-dihydro-1H-pyrazol-4-yl]hydrazin-1-ylidene}-6-oxocyclohexa-1,3-dien-1-yl)benzoic acid
(1,1'-Biphenyl)-3-carboxylic acid, 3'-((2Z)-(1-(3,4-dimethylphenyl)-1,5-dihydro-3-methyl-5-oxo-4H-pyrazol-4-ylidene)hydrazino)-2'-hydroxy-
3'-[2-[(2Z)-1-(3,4-Dimethylphenyl)-1,5-dihydro-3-methyl-5-oxo-4H-pyrazol-4-ylidene]hydrazinyl]-2'-hydroxy-[1,1'-biphenyl]-3-carboxylic acid
[1,1'-Biphenyl]-3-carboxylic acid, 3'-[2-[(2Z)-1-(3,4-dimethylphenyl)-1,5-dihydro-3-methyl-5-oxo-4H-pyrazol-4-ylidene]hydrazinyl]-2'-hydroxy-
[1,1'-Biphenyl]-3-carboxylic acid, 3'-[(2Z)-2-[1-(3,4-dimethylphenyl)-1,5-dihydro-3-methyl-5-oxo-4H-pyrazol-4-ylidene]hydrazinyl]-2'-hydroxy-
Eltrombopag
3'-[2-[(2Z)-1-(3,4-Dimethylphenyl)-1,5-dihydro-3-methyl-5-oxo-4H-pyrazol-4-ylidene]hydrazinyl]-2'-hydroxy-[1,1'-biphenyl]-3-carboxylic acid
3'-[2-[(2Z)-1-(3,4-Dimethylphenyl)-1,5-dihydro-3-methyl-5-oxo-4H-pyrazol-4-ylidene]hydrazinyl]-2'-hydroxy-[1,1'-biphenyl]-3-carboxylic acid Eltrombopag | [EINECS(EC#)]
610-469-9 | [Molecular Formula]
C25H22N4O4 | [MDL Number]
MFCD20926253 | [MOL File]
496775-61-2.mol | [Molecular Weight]
442.47 |
| Chemical Properties | Back Directory | [Melting point ]
242-244°C | [Boiling point ]
656.8±65.0 °C(Predicted) | [density ]
1.33 | [storage temp. ]
Keep in dark place,Inert atmosphere,Room temperature | [solubility ]
Soluble in DMSO (up to 55 mg/ml) or in ethanol (up to 14 mg/ml). | [form ]
solid | [pka]
-1.26±0.40(Predicted) | [color ]
Orange | [Stability:]
Stable for 2 years from date of purchase as supplied. Solutions in DMSO or ethanol may be stored at -20° for up to 3 months. | [InChI]
InChI=1S/C25H22N4O4/c1-14-10-11-19(12-15(14)2)29-24(31)22(16(3)28-29)27-26-21-9-5-8-20(23(21)30)17-6-4-7-18(13-17)25(32)33/h4-13,26,30H,1-3H3,(H,32,33)/b27-22- | [InChIKey]
XDXWLKQMMKQXPV-QYQHSDTDSA-N | [SMILES]
C1(C2=CC=CC(N/N=C3\C(=O)N(C4=CC=C(C)C(C)=C4)N=C\3C)=C2O)=CC=CC(C(O)=O)=C1 |
| Hazard Information | Back Directory | [Description]
ITP is an autoimmune disease in which antiplatelet antibodies accelerate the destruction of platelets. In addition, platelet production can be
impaired because the antiplatelet antibodies can also damage megakaryocytes. Although the thrombocytopenia of ITP can be severe, signs of
bleeding are usually only minor.
Eltrombopag is an oral, small-molecule, nonpeptide TPO-receptor agonist. It initiates TPO-receptor signaling by interacting with the
transmembrane domain of the receptor, thereby inducing proliferation
and differentiation of cells in the megakaryocytic lineage. Eltrombopag is
the second TPO-receptor agonist to reach the market behind romiplostim
(Nplate), a recombinant fusion protein launched in 2008. Both drugs are
specifically indicated for the treatment of thrombocytopenia in patients
with chronic ITP who have had an insufficient response to corticosteroids,
immunoglobulins, or splenectomy. Eltrombopag is supplied as the ethanolamine salt (also known as eltrombopag olamine).
The most common adverse reactions associated with eltrombopag were nausea, vomiting, mennorrhagia, myalgia, paresthesia and cataracts. Eltrombopag is derived in five synthetic steps starting from 2-bromo-6-nitroanisole, via Suzuki coupling reaction with 3-carboxyphenylboronic acid to a biphenyl intermediate, followed by cleavage of the methyl ether moiety with hydrobromic acid, and reduction of the nitro group to an amino group under catalytic hydrogenation conditions. Subsequently, the amino group is diazotized with sodium nitrite and hydrochloric acid, and the diazonium intermediate is condensed with 1-(3,4-dimethylphenyl)-3-methyl-2,5-dihydro-1H-pyrazol-5-one to produce eltrombopag. | [Chemical Properties]
Orange to Red Solid | [Originator]
Ligand Pharmaceuticals (US) | [Uses]
An agonist of the Thrombopoietin (Tpo) receptor, used as treatment for thrombocytopenia | [Uses]
Eltrombopag (SB-497115-GR, Promacta, Revolade) is a small molecule agonist of the c-mpl (TpoR) receptor with an IC50 of 0.69 μM for the inhibition of hERG K+ channel tail current. In preclinical studies, the compound was shown to interact selectively with | [Definition]
ChEBI: A hydrazine in which each nitrogen atom is substituted, one by a 3'-carboxy-2-hydroxy[1,1'-biphenyl]-3-yl group and the other by a 1-(3,4-dimethylphenyl)-3-methyl-5-oxo-1,5-dihydro-4H-pyrazol-4-ylidene group. A small molecule agonist of
the c-mpl (TpoR) receptor (the physiological target of the hormone thrombopoietin), it has been developed as a medication for conditions that lead to thrombocytopenia (abnormally low platelet counts). | [Brand name]
Promacta | [Clinical Use]
Thrombopoetin receptor agonist:
Treatment of chronic immune idiopathic
thrombocytopenic purpura (ITP)
Chronic hepatitis C associated thrombocytopenia
(HCV)
Severe aplastic anaemia | [Synthesis]
The synthesis of Eltrombopag is as follows:
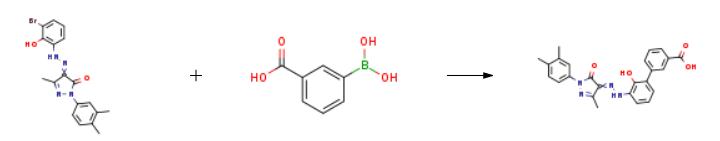
At room temperature, add 8.02g (20.0mmol) compound of formula V, 3.98g (24.0mmol) 3-carboxyphenylboronic acid, 8.48g (80.0mmol) sodium carbonate, 1.46g (2.0mmol) PdCl2dppf into a 250mL three-necked flask, 80mL ethanol, 65mL water, nitrogen protection, heated to 80, reacted for 10h, cooled to room temperature, added 60mL water, filtered, the filtrate was adjusted to pH 1-2 with 2M hydrochloric acid, a red solid was precipitated, filtered, and the filter cake was ethanol/water ( v/v=1:1, 40mL) beating, filtering, and drying to obtain 7.58g, the yield is 85.8%, and the purity is 99.40%.
 | [Drug interactions]
Potentially hazardous interactions with other drugs
Ciclosporin: concentration of eltrombopag reduced.
Statins: increased rosuvastatin concentration, may
need to reduce rosuvastatin dose. | [Metabolism]
Mainly hepatically metabolised through cleavage,
oxidation by cytochrome P450 isoenzymes CYP1A2
and CYP 2C8 and conjugation with glucuronic acid,
glutathione, or cysteine.
Approximately 31% of a dose is eliminated in the
urine as metabolites, and about 59% in the faeces (20%
unchanged). | [References]
1) Xie?et al. (2018), Pharmacological characterization of eltrombopag, a novel orally active human thrombopoietin receptor agonist; J. Cell. Mol. Med.,?22?5367
2) Erickson-Miller?et al.?(2008),?Preclinical activity of eltrombopag (SB-497115), an oral, nonpeptide thrombopoietin receptor agonist; Stem Cells,?27?424
3) Alvarado?et al.?(2019),?Eltrombopag maintains human hematopoietic stem and progenitor cells under inflammatory conditions mediated by IFN-γ; Blood,?133?2043
4) Guenther?et al.?(2019),?Eltrombopag promotes DNA repair in human hematopoietic stem and progenitor cells; Exp. Hematol.?73?1
5) Vlachodimitropoulou?et al.?(2017),?Eltrombopag: a powerful chelator of cellular or extracellular iron (II) alone or combined with a second chelator; Blood,?130?1923
6) Kao?et al.?(2018),?Thrombopoietin receptor-independent stimulation of hematopoietic stem cells by eltrombopag; Science Transl. Med.,?10?eaas9563 |
|
|