| Identification | More | [Name]
3-Hydroxy-4-methoxybenzyl alcohol | [CAS]
4383-06-6 | [Synonyms]
3-HYDROXY-4-METHOXYBENZYL ALCOHOL
ISOVANILLYL ALCOHOL
RARECHEM AL BD 0068
4-Methoxy-3-hydroxy benzyl alcohol
5-(Hydroxymethyl)-2-methoxyphenol
Benzenemethanol, 3-hydroxy-4-methoxy-
2-Methoxy-5-(hydroxymethyl)phenol
3-Hydroxy-4-methoxybenzenemethanol | [EINECS(EC#)]
224-489-7 | [Molecular Formula]
C8H10O3 | [MDL Number]
MFCD00004644 | [Molecular Weight]
154.16 | [MOL File]
4383-06-6.mol |
| Safety Data | Back Directory | [Hazard Codes ]
Xi | [Risk Statements ]
R36/37/38:Irritating to eyes, respiratory system and skin . | [Safety Statements ]
S22:Do not breathe dust .
S24/25:Avoid contact with skin and eyes .
S36:Wear suitable protective clothing .
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice . | [WGK Germany ]
3
| [HS Code ]
29094990 |
| Hazard Information | Back Directory | [Chemical Properties]
White crystalline | [Uses]
3-Hydroxy-4-methoxybenzyl alcohol(4383-06-6) was used in preparation of 2-hydroxymethyl-5-methoxy-2,5-cyclohexadiene-1,4-dione by oxidation with Fremy′s salt.
| [Synthesis]
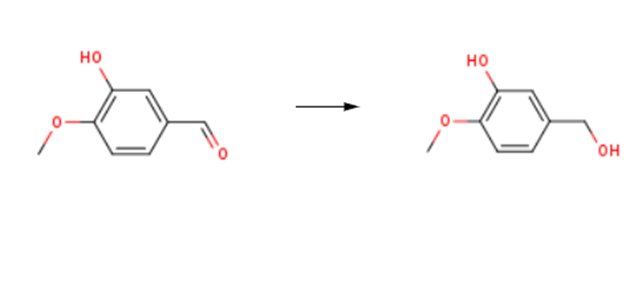
3-Hydroxy-4-methoxybenzyl alcohol(4383-06-6) is synthesised using isovanillin as raw material by chemical reaction. The specific synthesis steps are as follows:
In a round-bottom flask equipped with magnetic stirrer was added 15 sodium borohydride (0.2mmol; 0.01g) to the solution of 9 1 (0.2mmol; 0.03g) prepared in ethanol (1mL). The mixture was stirred at room temperature for 30min. The consumption of all starting material was detected by thin layer chromatography (TLC); the solvent was evaporated under reduced pressure. Subsequently, 10mL of saturated NaCl solution was added and then extracted with ethyl acetate (3×10mL). The organic phase was dried over anhydrous MgSO4, filtered and evaporated, providing 0.03g of colorless oil, 90% yield of 3-Hydroxy-4-methoxybenzyl alcohol.
|
|
|