| Identification | More | [Name]
Nimustine | [CAS]
42471-28-3 | [Synonyms]
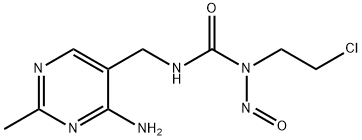
1-(4-AMINO-2-METHYL-5-PYRIMIDINYL)-METHYL-3-(2-CHLOROETHYL)-3-NITROSOUREA HYDROCHLORIDE
1-((4-amino-2-methylpyrimidin-5-yl)methyl)-3-(2-chloroethyl)-3-nitrosourea
1-((4-amino-2-methylpyrimidin-5-yl)methyl)-3-(2-chloroethyl)-3-nitrosourea hydrochloride
3-[(4-amino-2-methyl-pyrimidin-5-yl)methyl]-1-(2-chloroethyl)-1-nitroso-urea hydrochloride
ACNU
N'-[(4-AMINO-2-METHYL-5-PYRIMIDINYL)METHYL]-N-(2-CHLORETHYL)-N-NITROSOUREA
N'-[(4-AMINO-2-METHYL-5-PYRIMIDINYL)METHYL]-N-(2-CHLOROETHYL)-N-NITROSOUREA HYDROCHLORIDE
NIMUSTINE
NIMUSTINE HCL
NIMUSTINE HYDROCHLORIDE
3-(4-amino-2-methyl-5-pyrimidinyl)methyl-1-(2-chloroethyl)-1-nitrosourea
n’-((4-amino-2-methyl-5-pyrimidinyl)methyl)-n-(2-chloroethyl)-n-nitroso-ure
Nimustin
NITROSOUREA
NIFUMICACID
1-[(4-Amino-2-methyl-5-pyrimidinyl)methyl]-3-(2-chloroethyl)-3-nitrosourea
3-[(4-Amino-2-methylpyrimidin-5-yl)methyl]-1-(2-chloroethyl)-1-nitrosourea | [EINECS(EC#)]
255-838-1 | [Molecular Formula]
C9H14Cl2N6O2 | [MDL Number]
MFCD01676942 | [Molecular Weight]
309.15 | [MOL File]
42471-28-3.mol |
| Safety Data | Back Directory | [Hazard Codes ]
T | [Risk Statements ]
R25:Toxic if swallowed. | [Safety Statements ]
S36/37/39:Wear suitable protective clothing, gloves and eye/face protection .
S45:In case of accident or if you feel unwell, seek medical advice immediately (show label where possible) . | [RIDADR ]
UN 2811 6.1/PG 3
| [WGK Germany ]
3
| [RTECS ]
YR8450000
|
| Hazard Information | Back Directory | [Originator]
Nidran,Sankyo,Japan,1979 | [Definition]
ChEBI: An organochlorine compound that is urea in which the two hydrogens on one of the amino groups are replaced by nitroso and 2-chloroethyl groups and one hydrogen from the other amino group is replaced by a 4-amino-2-methylpyrimidin-5-ylmethyl] group. An anti
eoplastic agent especially effective against malignant brain tumors. | [Manufacturing Process]
0.4 g of sodium nitrite was added with stirring, at 0°C to 5°C, to a solution of
450 mg of 1-(2-chloroethyl)-3-[(2-methyl-4-aminopyridin-5-yl)methyl]urea in
8 ml of 5% hydrochloric acid, and the reaction mixture was then stirred at
0°C to 10°C for an additional 1.5 hours.
After completion of the reaction, the reaction mixture was made alkaline by
the addition of sodium carbonate, whereupon crystals separated out in situ.
The crystals were recovered by filtration, washed with water and then
recrystallized from 6 ml of ethanol, to give 0.1 g of the pale yellow pure
desired product having a decomposition point of 125°C. | [Therapeutic Function]
Antitumor, Antileukemic |
|
| Company Name: |
LGM Pharma
|
| Tel: |
1-(800)-881-8210 |
| Website: |
www.lgmpharma.com |
| Company Name: |
HBCChem, Inc.
|
| Tel: |
+1-510-219-6317 |
| Website: |
www.warehouse-sample-usa.com |
|