| Identification | More | [Name]
N-(5H-Purin-6-yl)benzamide | [CAS]
4005-49-6 | [Synonyms]
6-BENZAMIDOPURINE
6-BENZOYLAMINOPURINE
N-(5H-Purin-6-yl)benzamide
N6-BENZOYLADENINE
N6-BENZOYLAMINOPURINE
N-BENZOYLADENINE
N-BENZOYLAMINOPURINE
N-BZ-ADE
N-Benzolaminopurine
N-Benzoylaminopurine,99%
Benzamide, N-1H-purin-6-yl-
6-Benzoylaminopurin
N(sup 6)benzoyladenine | [EINECS(EC#)]
629-048-6 | [Molecular Formula]
C12H9N5O | [MDL Number]
MFCD00037927 | [Molecular Weight]
239.23 | [MOL File]
4005-49-6.mol |
| Chemical Properties | Back Directory | [Melting point ]
242-244°C | [density ]
1.494 g/cm3 | [storage temp. ]
2-8°C
| [Water Solubility ]
Insoluble in water | [form ]
powder to crystal | [pka]
11.98±0.20(Predicted) | [color ]
White to Almost white | [BRN ]
20585 | [InChI]
InChI=1S/C12H9N5O/c18-12(8-4-2-1-3-5-8)17-11-9-10(14-6-13-9)15-7-16-11/h1-7H,(H2,13,14,15,16,17,18) | [InChIKey]
QQJXZVKXNSFHRI-UHFFFAOYSA-N | [SMILES]
C(NC1=C2C(=NC=N1)NC=N2)(=O)C1=CC=CC=C1 | [CAS DataBase Reference]
4005-49-6(CAS DataBase Reference) |
| Safety Data | Back Directory | [Hazard Codes ]
Xn,Xi | [Risk Statements ]
R22:Harmful if swallowed.
R43:May cause sensitization by skin contact. | [Safety Statements ]
S36/37:Wear suitable protective clothing and gloves . | [WGK Germany ]
3
| [Hazard Note ]
Irritant | [HS Code ]
29335990 |
| Hazard Information | Back Directory | [Chemical Properties]
White solid | [Uses]
N6-Benzoyladenine is used in the organic synthesis of adenine derivative molecules such as bicyclic adenine nucleoside via a condensation reaction between L-threo-pentofuranose derivative 1 and 6-N-benzoyladenine and to produce oxy-peptide nucleic acids. | [Uses]
N-Benzoylaminopurine is used in the organic synthesis of adenine derivative molecules such as bicyclic adenine nucleoside via a condensation reaction between L-threo-pentofuranose derivative 1 and 6-N-benzoyladenine and to produce oxy-peptide nucleic acids.
| [Definition]
ChEBI: 6-Benzamidopurine is a member of purines. | [Biochem/physiol Actions]
N6-Benzoyladenine comprises of adenine moiety and is a potent inhibitor of bromodomain-containing protein 4 (BRD4). N6-Benzoyladenine modulates tumor necrosis factor α (TNF-α) levels. It elicits cytotoxicity in liver and ileum cancer cells and may serve as a potential candidate agent for cancer chemotherapy. | [Synthesis]
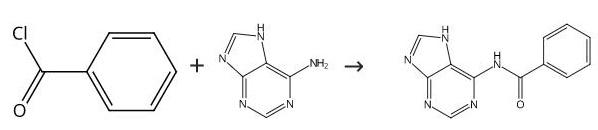
Benzoyl chloride (1.3 mL, 11 mmol) was added dropwise over 30 min to a stirred suspension of adenine (1.35 g, 10 mmol) in dry pyridine, and stirring was continued at 100 °C for a further 3 h, and the reaction mixture was allowed to stand overnight at room temperature. The reaction was quenched with methanol and the solvents were removed under reduced pressure. The residue was triturated in hot isopropanol and dried in vacuo to give 8 as a white solid: yield 2.15 g (90%). MS (+ESI): m/z 240 [M+H]+; 1H NMR (400 MHz, d-DMSO): δ11.50 (s, 1H), 8.74 (s, 1H), 8.52 (s, 1H), 8.11 (d, 2H, J = 7.2 Hz), 7.66 (t, 1H, J = 7.2 Hz), 7.58 (t, 2H, J = 7.2 Hz).
 |
|
|