| Identification | More | [Name]
Trimethoxy[2-(7-oxabicyclo[4.1.0]hept-3-yl)ethyl]silane | [CAS]
3388-04-3 | [Synonyms]
1-[2-(TRIMETHOXYSILYL)ETHYL]CYCLOHEXANE-3,4-EPOXIDE
2-(3,4-EPOXYCYCLOHEXYL)ETHYLTRIMETHOXYSILANE
2-(7-OXABICYCLO[4.1.0]HEPT-3-YL)ETHYL-TRIMETHOXYSILANE
3-(2-TRIMETHOXYSILYLETHY)CYCLOHENE OXIDE
B-(3,4-EPOXYCYCLOHEXYL)ETHYLTRIMETHOXYSILANE
BETA-(3,4-EPOXYCYCLOHEXYL)-ETHYLTRIMETHOXYSILANE
TRIMETHOXY[2-(7-OXABICYCLO[4.1.0]HEPT-3-YL)ETHYL]SILANE
TRIMETHOXY(2-(OXABICYCLO(4.1.0)HEPT-3-YL)) ETHYLSILANE
((Epoxycyclohexyl)ethyl)trimethoxy silane
(3,4-epoxycyclohexyl)ethyltrimethoxysilane
2-(3,4-Epoxycyclohexyl) ethyltriacetoxysilane
3,4-Epoxycyclohexylethyltrimethoxysilane
3-[2-(Trimethoxysilyl)Ethyl]-7-oxabicyclo[4.1.0]heptane
4-[2-(Trimethoxysilyl)ethyl]-7-oxabicyclo[4.1.0]heptane
A 186
A 186 (Heterocycle)
a186(couplingagent)
beta-(3,4-epoxycyclohexyl)ethyltrimethoxy-silan
CE6250
e6250 | [EINECS(EC#)]
222-217-1 | [Molecular Formula]
C11H22O4Si | [MDL Number]
MFCD00014485 | [Molecular Weight]
246.38 | [MOL File]
3388-04-3.mol |
| Safety Data | Back Directory | [Hazard Codes ]
T | [Risk Statements ]
R45:May cause cancer. | [Safety Statements ]
S53:Avoid exposure-obtain special instruction before use .
S23:Do not breathe gas/fumes/vapor/spray (appropriate wording to be specified by the manufacturer) .
S36/37/39:Wear suitable protective clothing, gloves and eye/face protection .
S45:In case of accident or if you feel unwell, seek medical advice immediately (show label where possible) . | [WGK Germany ]
3
| [RTECS ]
VV4000000
| [F ]
3-10 | [TSCA ]
Yes | [HS Code ]
29319090 | [Toxicity]
LD50 oral in rat: 8mL/kg |
| Hazard Information | Back Directory | [Uses]
Trimethoxy[2-(7-oxabicyclo[4.1.0]hept-3-yl)ethyl]silane was used as an adhesion promoter during the fabrication of:
- a photoacid generator activated by two photon excitation.
- nanoscale polymeric structures (width: 65 nm) using 520 nm femtosecond pulse excitation.
Trimethoxy[2-(7-oxabicyclo[4.1.0]hept-3-yl)ethyl]silane may be used as a precursor to form SiCOH film via sol-gel. The silane may be used to functionalize alumina nanoparticles towards the fabrication of polyamide 12/alumina nanocomposites. 4 Basalt fibers were functionalized with this silane and its consequent effect on the fabrication of basalt fiber–epoxidized vegetable oil matrix composite materials was analyzed. | [Uses]
Trimethoxy[2-(7-oxabicyclo[4.1.0]hept-3-yl)ethyl]silane may be used as a precursor to form SiCOH film via sol-gel. The silane may be used to functionalize alumina nanoparticles towards the fabrication of polyamide 12/alumina nanocomposites. 4 Basalt fibers were functionalized with this silane and its consequent effect on the fabrication of basalt fiber–epoxidized vegetable oil matrix composite materials was analyzed.Trimethoxy[2-(7-oxabicyclo[4.1.0]hept-3-yl)ethyl]silane can be used as a silane based coupling agent to functionalize a variety of substrates. It modifies the surface to improve the dispersion of nanoparticles. It can be used as an adhesion promoter by treating the precursor material with epoxy silanes. | [Application]
Trimethoxy[2-(7-oxabicyclo[4.1.0]hept-3-yl)ethyl]silane was used as an adhesion promoter during the fabrication of:a photoacid generator activated by two photon excitation.nanoscale polymeric structures (width: 65 nm) using 520 nm femtosecond pulse excitation. | [Flammability and Explosibility]
Nonflammable | [Synthesis]
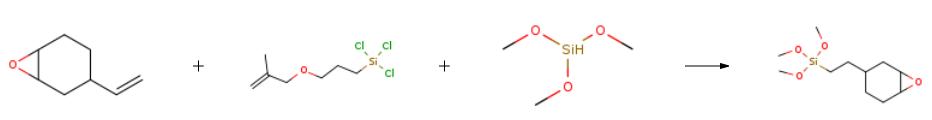
The apparatus of Example B was charged with 148.8 g (1.2 mol) of 1-vinyl-3,4-epoxycyclohexane, 1.3 g of a carboxylic acid promoter, and 0.15 ml of 10% chloroplatinic acid catalyst solution. The flask contents were heated to 89?? C. and dropwise addition of 122.8 g {[1.0 mol) of trimethoxysilane was begun. The reaction temperature was controlled at 90??-95?? C. with an ice bath. Reaction was maintained at that temperature for half an hour after completion of addition, which took 18 minutes. Analysis by gas chromatography showed a yield of 90% of 2-(3,4-epoxycyclohexyl)ethyltrimethoxysilane. This example demonstrates a standard preparation of 2-(3,4-epoxycyclohexyl)ethyltrimethoxysilane using commercial chloroplatinic acid. |
|
|