| Identification | More | [Name]
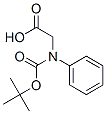
Boc-D-Phenylglycine | [CAS]
33125-05-2 | [Synonyms]
BOC-L-ALPHA-PHENYLGLYCINE
BOC-L-PHENYLGLYCINE
BOC-L-PHG
BOC-L-PHG-OH
BOC-PHENYLGLYCINE
BOC-(PHENYL)GLY-OH
BOC-PHG-OH
N-ALPHA-T-BOC-L-PHENYLGLYCINE
N-ALPHA-T-BUTOXYCARBONYL-T-PHENYLGLYCINE
N-ALPHA-(T-BUTYLOXYCARBONYL)-L-PHENYLGLYCINE
N-ALPHA-TERT-BUTYLOXYCARBONYL-L-PHENYLGLYCINE
N-BOC-L-PHENYLGLYCINE
RARECHEM EM WB 0179
(S)-TERT-BUTOXYCARBONYLAMINO-PHENYL-ACETIC ACID
Boc-D-Phg-OH~N-(tert-Butoxycarbonyl)-D-phenylglycine
N-tert-Butoxycarbonyl-D-phenylglycine
Boc-D-Phenylglycine
BOC-3-CHLORO-PHENYLGLYCINE
Boc-(R)-2-aminobenzeneacetic acid
N-(tert-Butoxycarbonyl)-D-2-phenylglycine | [EINECS(EC#)]
1533716-785-6 | [Molecular Formula]
C13H17NO4 | [MDL Number]
MFCD00065588 | [Molecular Weight]
251.28 | [MOL File]
33125-05-2.mol |
| Chemical Properties | Back Directory | [Melting point ]
88-91 °C | [alpha ]
-142 º (c=1% in ethanol) | [Boiling point ]
407.2±38.0 °C(Predicted) | [density ]
1.182±0.06 g/cm3(Predicted) | [refractive index ]
-140 ° (C=1, EtOH) | [storage temp. ]
Store at RT. | [form ]
Crystalline Powder | [pka]
3.51±0.10(Predicted) | [color ]
White | [optical activity]
[α]20/D 141.0±5.0°, c = 1% in ethanol | [Water Solubility ]
Insoluble in water. Slightly soluble in DMSO and methanol. | [BRN ]
3033982 | [CAS DataBase Reference]
33125-05-2(CAS DataBase Reference) |
| Safety Data | Back Directory | [Safety Statements ]
S24/25:Avoid contact with skin and eyes . | [WGK Germany ]
3
| [HazardClass ]
IRRITANT | [HS Code ]
29242990 |
| Hazard Information | Back Directory | [Chemical Properties]
White powder | [Uses]
It is a reagent of choice for assignment of absolute configuration of chiral primary amines by 1H NMR, giving better results than Mosher's acid ((R)-(+)-?-Methoxy-?-(trifluoromethyl)-phenyl-acetic acid. αR)-α-[[(1,1-Dimethylethoxy)carbonyl]amino]-benzeneacetic Acid is D-(+)-2-Phenylglycine with Boc protecting group. (αR)-α-[[(1,1-Dimethylethoxy)carbonyl]amino]-benzeneacetic Acid is used as part of a catalyst combination to catalyze regioselective [4 + 2] cycloadditions of β-substituted cyclic enones and polyconjugated malononitriles. It can also be used to catalyze stereoselective preparation of polyfunctional nitrocyclohexene carboxaldehydes. |
|
|