| Identification | Back Directory | [Name]
Butyldi-1-adamantylphosphine | [CAS]
321921-71-5 | [Synonyms]
CATACXIUM A
cataCXium(R) A
Catalyst 1 ,95%
cataCXium(R) A 95%
Di(adamantan-1-yl)
BUTYLDI-1-ADAMANTYLPHOSPHINE
nbutyl-di(1-adamantyl)phosphine
Di(1-adamantyl)-n-butylphosphine
Bis(1-adaMantyl)-butyl-phosphane
Bis(adamant-1-yl)(butyl)phosphine
Bis(1-AdaMantyl)-n-Butylphosphine
Butyldi-1-adamantylphosphine ,95%
Di(adaMantan-1-yl)(butyl)phosphine
bis(adaMantan-1-yl)(butyl)phosphane
Di(1-adamantyl)-n-butylphosphine,95%
Di(1-adamantyl)-n-butylphosphine 95%
Butyldi-1-adamantylphosphine,min. 95%
BUTYLDI-1-ADAMANTYLPHOSPHINE [CATACXIUM A]
Butylbis(tricyclo[3.3.1.13,7]dec-1-yl)phosphine
Butyldi-1-adamantylphosphine,min.95%[cataCXiumA]
Phosphine,butylbis(tricyclo[3.3.1.13,7]dec-1-yl)-
Butyldi-1-adamantylphosphine, min. 95% [cataCXium(R) A] | [EINECS(EC#)]
1312995-182-4 | [Molecular Formula]
C24H39P | [MDL Number]
MFCD05861606 | [MOL File]
321921-71-5.mol | [Molecular Weight]
358.54 |
| Chemical Properties | Back Directory | [Melting point ]
100°C | [Boiling point ]
449.6±12.0 °C(Predicted) | [storage temp. ]
Inert atmosphere,Room Temperature | [form ]
Powder | [color ]
white to yellow | [Sensitive ]
air sensitive | [BRN ]
8726448 | [InChI]
InChI=1S/C24H39P/c1-2-3-4-25(23-11-17-5-18(12-23)7-19(6-17)13-23)24-14-20-8-21(15-24)10-22(9-20)16-24/h17-22H,2-16H2,1H3 | [InChIKey]
HTJWUNNIRKDDIV-UHFFFAOYSA-N | [SMILES]
P(CCCC)(C12CC3CC(CC(C3)C1)C2)C12CC3CC(CC(C3)C1)C2 | [CAS DataBase Reference]
321921-71-5 |
| Hazard Information | Back Directory | [Uses]
suzuki reaction | [Physical properties]
Butyldi-1-adamantylphosphine is a white to yellow solid with a melting
point of 100°C and an estimated boiling point of 449.6±12.0°C. Store at
room temperature, it is air sensitive. | [General Description]
Sold in collaboration with Solvias AG | [reaction suitability]
reagent type: ligand
reaction type: Arylations
reagent type: ligand
reaction type: Buchwald-Hartwig Cross Coupling Reaction
reagent type: ligand
reaction type: C-C Bond Formation
reagent type: ligand
reaction type: Cross Couplings
reagent type: ligand
reaction type: Heck Reaction
reagent type: ligand
reaction type: Sonogashira Coupling
reagent type: ligand
reaction type: Suzuki-Miyaura Coupling | [Synthesis]
Phosphonium salt (2.2 mmol) was added to a cooled solution (- 78 ??C) of
Et3N
(4.44 g, 44 mmol) in di-n-butyl ether (20 mL). The reaction mixture was
stirred at -78 ??C for 5 h and then allowed to warm gradually to r.t.
The solvent was removed under vacuum and the residue was dissolved in
degassed EtOH (5 mL). After stirring for 15 min, the solid was filtered
off and dried to yield the desired phosphine, which can be further
purified by crystallization from EtOH. Butyldi-1-adamantylphosphine,
yield 90%.
31P NMR (C6D6)|?: 24.9. Mp 108-110??C. IR (KBr): 3425 (m, br),
2952 (s), 2847 (s), 2847 (s), 2675 (w), 1446 cm-1 (m). 1H NMR (250 MHz,
C6D6): |? = 0.96 (3 H, t, 3JH, H = 7.3 Hz, CH3), 1.35-2.03 (36 H, m,
adamantyl-30H, butyl-6H). 13C NMR (62 MHz, C6D6): |? = 41.3 (d, 2JC,P =
11.3 Hz, C-2), 37.4 (C-4), 36.1 (d, 1JC,P = 23.5 Hz, C-1), 33.9 (d,
1JC,P = 26.2 Hz, butyl-|á -CH2), 29.1 (d, 3JC,P = 7.6 Hz, C-3), 24.9 (d,
2JC,P = 13.1 Hz, butyl-|? -CH2), 17.1 (d, 3JC,P = 21.6 Hz, butyl-|? -CH2),
14.3 (butyl-CH3). MS (EI, 70 eV): m/z (%) = 358 (M+, 60), 135 (Ad+,
100). |
| Questions And Answer | Back Directory | [Reaction]
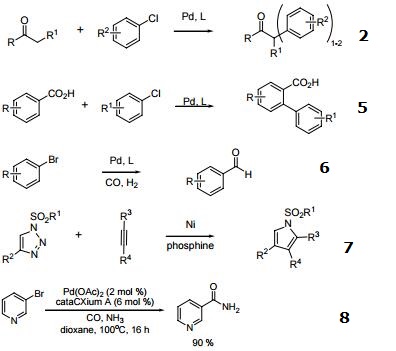
- Ligand for the Pd-catalyzed Suzuki coupling reaction.
- Ligand for the Pd-catalyzed formation of α-aryl ketones.
- Ligand for the Pd-catalyzed aminations
- Ligand for the Pd-catalyzed Heck reaction.
- Ligand used for arylation of benzoic acids.
- Ligand for the formylation of aryl bromides.
- Ni-catalyzed denitrogenative alkyne insertion reactions of triazoles.
- Ligand for palladium-catalyzed aminocarbonylation of aryl halides
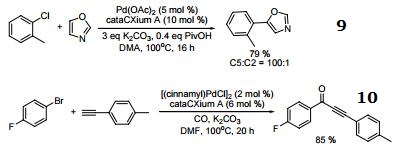
- Palladium-catalyzed direct arylation of oxazole at C-5 with aryl bromides, chlorides, and triflates
- Palladium-catalyzed carbonylative sonogashira coupling of aryl bromides.
|
|
|