| Identification | More | [Name]
FENOPROFEN | [CAS]
31879-05-7 | [Synonyms]
FENOPROFEN
(.+/-.)-m-Phenoxyhydratropic acid
(+-)-2-(3-phenoxyphenyl)propionicacid
(+-)-m-phenoxyhydrotropicacid
2-(3-Phennoxyphenyl)propanoicacid
2-(3-Phenoxyphenyl)propanoic acid
alpha-dl-2-(3-phenoxyphenyl)propionicacid
alpha-methyl-3-phenoxybenzeneaceticacid
a-Methyl-3-phenoxybenzeneaceticacid
Benzeneacetic acid, alpha-methyl-3-phenoxy-, (.+/-.)-
dl-2-(3-phenoxyphenyl)propionicacid
Fenopolfen
Hydratropic acid, m-phenoxy-, (.+/-.)-
lilly53838
Lilly-53858
FENOPROFEN CALCIUM USP(CRM STANDARD)
2-[3-(Phenyloxy)phenyl]propanoic acid | [EINECS(EC#)]
250-850-3 | [Molecular Formula]
C15H14O3 | [MDL Number]
MFCD00072027 | [Molecular Weight]
242.27 | [MOL File]
31879-05-7.mol |
| Hazard Information | Back Directory | [Originator]
Fenopron,Dista,UK,1974 | [Uses]
antiinflammatory | [Uses]
Fenoprofen is used in treating symptoms of rheumatoid arthritis and osteoarthritis;
however, fenoprofen exhibits a number of undesirable side effects. | [Definition]
ChEBI: Propanoic acid in which one of the hydrogens at position 2 is substituted by a 3-phenoxyphenyl group. A non-steroidal anti-inflammatory drug, the dihydrate form of the calcium salt is used for the management of mild to moderate pain and for the relief of p
in and inflammation associated with disorders such as arthritis. It is pharmacologically similar to aspirin, but causes less gastrointestinal bleeding. | [Indications]
Fenoprofen (Nalfon) is chemically and pharmacologically
similar to ibuprofen and is used in the treatment
of rheumatoid arthritis, osteoarthritis, and mild to
moderate pain. GI effects such as dyspepsia and pain
are most common, although dizziness, pruritus, and palpitations
may occur. GI bleeding, sometimes severe, has
been reported, and interstitial nephritis has been rarely
associated with this drug. Concomitant administration
of aspirin decreases the biological half-life of fenoprofen
by increasing the metabolic clearance of hydroxylated
fenoprofen. Chronic administration of phenobarbital
also decreases the drug’s half-life. | [Manufacturing Process]
3-Phenoxyacetophenone: A mixture consisting of 908 grams (6.68 mols) of
m-hydroxyacetophenone, 4,500 grams (28.6 mols) of bromobenzene, 996
grams (7.2 mols) of anhydrous potassium carbonate, and 300 grams of
copper bronze was heated under reflux with stirring until water evolution was
complete, using a Dean-Stark water separator. The mixture was then stirred
and refluxed for 24 hours. After cooling to room temperature, the reaction
was diluted with an equal volume of CHCl3 and filtered. The filtrate was
washed with 5% HCl, then with 5% NaOH, with water, dried over Na2SO4 and
evaporated in vacuo. The residual oil was distilled through a 15 cm Vigreux
column, yielding 918 grams of 3-phenoxy-acetophenone, BP 120° to 121°C
(0.09 mm).
α-Methyl-3-Phenoxybenzyl Alcohol: A stirred solution of 700 grams of mphenoxyacetophenone
in 3,000 ml anhydrous methanol was cooled to 0°C in
an ice-acetone bath. Sodium borohydride, 136 grams (3.6 mols) was added to
this solution in small portions at such a rate that the temperature never rose
above 10°C. After borohydride addition was complete, the reaction mixture
was allowed to warm to room temperature and stirred for 18 hours. It was
then stirred and refluxed for 8 hours. About 400 ml of methanol was distilled
out and the remaining solution was evaporated to about one-third its original
volume in vacuo and poured into ice water. This mixture was extracted twice
with ether, acidified with 6 N HCl, and again extracted with ether. The ether
extracts were combined, washed with saturated NaCl solution, dried over
anhydrous sodium sulfate, and evaporated in vacuo. The residual oil was
distilled through a 15 cm Vigreux column, yielding 666 grams of α-methyl-3-
phenoxybenzyl alcohol, BP 132° to 134°C (0.35 mm), nD
25 = 1.5809.
α-Methyl-3-Phenoxybenzyl Bromide: A stirred solution of 1,357 grams of α-
methyl-3-phenoxybenzyl alcohol in 5,000 ml anhydrous CCl4 (predriedover
molecular sieve) was cooled to 0°C. To this was added 1,760 grams
PBr3,stirring and cooling being maintained at such a rate that the temperature
remained at 0° to 5°C, during the addition. The reaction mixture was then
allowed to warm to room temperature and was stirred at room temperature
overnight (ca 12 hours). The reaction mixture was then poured into ice water
and the organic phase separated. The aqueous phase was extracted with CCl4
and the combined extracts were washed three times with water, dried over
anhydrous sodium sulfate and evaporated to dryness in vacuo to yield 1,702
grams of α-methyl-3-phenoxybenzyl bromide as a heavy viscous oil,
nD
25=1.5993.
2-(3-Phenoxyphenyl)Propionitrile: A well-stirred suspension of 316 grams of
98% sodium cyanide in 5,000 ml of anhydrous dimethyl sulfoxide (previously
dried over molecular sieve) was warmed to 55° to 60°C and maintained at
this temperature while 1,702 grams of α-methyl-3-phenoxybenzyl bromide
was slowly added. After the bromide addition was completed, the temperature
was raised to 75°C and the mixture stirred at this temperature for 1.5 hours.
The mixture was then allowed to cool to room temperature and was stirred
overnight at room temperature and then poured into ice water. The resulting
aqueous suspension was extracted twice with ethyl acetate, and then with
ether. The organic extract was washed twice with a sodium chloride solution,
once with water, and dried over anhydrous sodium sulfate. Evaporation of the solvent in vacuo left an oily residue which was distilled through a 15 cm
Vigreux column to yield 1,136 grams of 2-(3-phenoxyphenyl)propionitrile, BP
141° to 148°C (0.1 mm), nD
25 = 1.5678.
2-(3-Phenoxyphenyl)Propionic Acid: A mixture of 223 grams of 2-(3-
phenoxyphenyl)propionitrile and 400 grams of sodium hydroxide in 1,600 ml
of 50% ethanol was refluxed with stirring for 72 hours. After cooling to room
temperature, the reaction mixture was poured into ice water. The resulting
solution was washed with ether, acidifed with concentrated HCl, and extracted
with ether. The ether extract was washed with water, dried over anhydrous
sodium sulfate, and evaporated to dryness in vacuo. The residual oil was
distilled to yield 203.5 grams (84%) of 2-(3-phenoxyphenyl)propionic acid as
a viscous oil; BP 168° to 171°C (0.11 mm), nD
25 = 1.5742. | [Brand name]
Nalfon (Dista); Nalfon (Pedinol). | [Therapeutic Function]
Antiinflammatory | [General Description]
Fenoprofen (Nalfon), is rapidly absorbed orally, reachespeak plasma levels within 2 hours, and has a short plasmahalf-life (3 hours). It is highly protein bound, just like theother NSAIDs, thus caution is needed when it is used concurrentlywith other medications including hydantoins, sulfonamides,and sulfonylureas. It is recommended for RAand OA, at an oral dose of 300 to 600 mg for 3 or 4 timesper day, but not exceeding 3 g/d to avoid any serious side effects.It should be noted that in a comparison study of allNSAIDs, fenoprofen is the one that has been most closelyassociated with a rare acute interstitial nephritis.188 For mildto moderate pain relief, the recommended dosage is 200 mggiven every 4 to 6 hours, as needed. | [Clinical Use]
Clinical use
NSAID and analgesic | [Synthesis]
Fenoprofen, 2-(3-phenoxyphenyl)propionic acid (3.2.32), is synthesized from
3-hydroxyacetophenone, which is esterfied by bromobenzene in the presence of potassium
carbonate and copper filings, forming 3-phenoxyacetophenone (3.2.28). The carbonyl
group of the resulting product is reduced by sodium borohydride and the resulting alcohol
(3.2.29) is brominated by phosphorous tribromide. The reaction of the resulting bromo
derivative (3.2.20) with sodium cyanide gives 2-(3-phenoxyphenyl)propionitrile (3.2.31),
which is hydrolyzed into the desired fenoprofen (3.2.32) [102,103].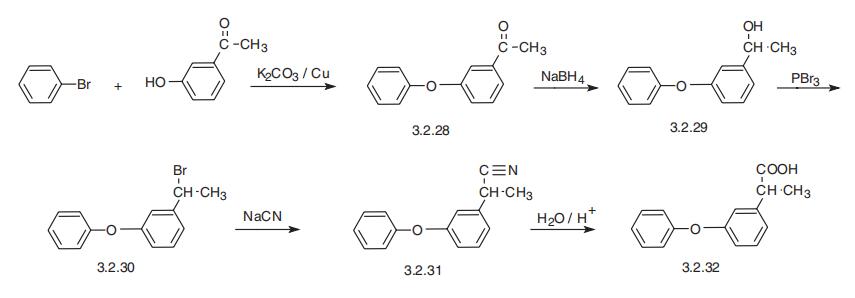
| [Drug interactions]
Potentially hazardous interactions with other drugs
ACE inhibitors and angiotensin-II antagonists:
increased risk of hyperkalaemia and nephrotoxicity;
reduced hypotensive effect.
Analgesics: avoid concomitant use with other
NSAIDs or aspirin; avoid concomitant use with
ketorolac (increased side effects and haemorrhage).
Antibacterials: possibly increased risk of convulsions
with quinolones.
Anticoagulants: effects of coumarins and
phenindione enhanced; possibly increased risk of
bleeding with heparin, dabigatran and edoxaban -
avoid long term use with edoxaban.
Antidepressants: increased risk of bleeding with
SSRIs or venlafaxine.
Antidiabetics: effects of sulphonylureas enhanced.
Antiepileptics: possibly enhanced effect of phenytoin.
Antivirals: concentration possibly increased by
ritonavir; increased risk of haematological toxicity
with zidovudine.
Ciclosporin: may potentiate nephrotoxicity.
Cytotoxics: reduced excretion of methotrexate;
increased risk of bleeding with erlotinib.
Diuretics: increased risk of nephrotoxicity;
antagonism of diuretic effect; hyperkalaemia with
potassium-sparing diuretics.
Lithium: excretion reduced.
Pentoxifylline: increased risk of bleeding.
Tacrolimus: increased risk of nephrotoxicity | [Metabolism]
Molecular weight (daltons) 558.6 (as calcium salt)
% Protein binding >99
% Excreted unchanged in urine 2-5
Volume of distribution (L/kg) 0.10
Half-life - normal/ESRF (hrs) 3 / Unchanged |
|
|