| Identification | More | [Name]
2-Amino-N-(2-chloro-6-methylphenyl)thiazole-5-carboxamide | [CAS]
302964-24-5 | [Synonyms]
2-AMINO-N-(2-CHLORO-6-METHYLPHENYL)THIAZOLE-5-CARBOXAMIDE | [EINECS(EC#)]
1806241-263-5 | [Molecular Formula]
C11H10ClN3OS | [MDL Number]
MFCD10000630 | [Molecular Weight]
267.73 | [MOL File]
302964-24-5.mol |
| Chemical Properties | Back Directory | [Melting point ]
208.0 to 212.0 °C | [Boiling point ]
362.0±37.0 °C(Predicted) | [density ]
1.474±0.06 g/cm3(Predicted) | [storage temp. ]
Keep in dark place,Inert atmosphere,2-8°C | [solubility ]
DMSO (Slightly), Methanol (Sparingly) | [form ]
powder to crystal | [pka]
11.09±0.70(Predicted) | [color ]
White to Light yellow | [InChI]
InChI=1S/C11H10ClN3OS/c1-6-3-2-4-7(12)9(6)15-10(16)8-5-14-11(13)17-8/h2-5H,1H3,(H2,13,14)(H,15,16) | [InChIKey]
VVOXTERFTAJMAA-UHFFFAOYSA-N | [SMILES]
S1C(C(NC2=C(C)C=CC=C2Cl)=O)=CN=C1N | [CAS DataBase Reference]
302964-24-5(CAS DataBase Reference) |
| Hazard Information | Back Directory | [Uses]
2-Amino-N-(2-chloro-6-methylphenyl)-5-thiazolecarboxamide(302964-24-5), is a precursor in the synthesis of Dasatinib (D193600), a protein tyrosine kinase inhibitor, and also for 2-Amino-thiazole-5-carboxylic Acid Phenylamide Derivatives, used as potent and selective anti-tumor drugs.
| [Synthesis]
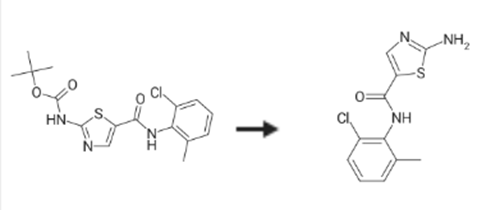
2-Amino-N-(2-chloro-6-methylphenyl)thiazole-5-carboxamide(302964-24-5) is synthesised using Tert-butyl (5-((2-chloro-6-methylphenyl)carbamoyl)thiazol-2-YL)carbamate as a raw material by chemical reaction. The specific synthesis steps are as follows:
To the stirred solution of trifluoroacetic acid (TFA) (250.0 mL) added tert-butyl (5-((2-chloro-6-methylphenyl)carbamoyl)thiazol-2-yl)carbamate 23 (25.0 g, 0.07 mol) and stirred for 2h at 25-30°C. Reaction completion was confirmed by TLC, distilled the TFA and the residue was diluted with ethyl acetate (EtOAc),(250.0 mL). Ethyl acetate layer was washed with saturated NaHCO3 solution (2 X 25.0 mL), water, followed bysaturated NaCl solution (125.0 mL). Ethyl acetate layer was dried over Na2SO4 and concentrated. The residual mass on treatment with methyl tert-butyl ether (250.0) gave 9 (16.2 g, 89percent) [11].IR (KBr, cm-1): 3382.4, 3284.85 (N-H), 1627.02 (C=O), 1644; 1H NMR spectrum (400 MHz, DMSO-d6), δ, ppm(J, Hz): 9.63 (s, 1H, thiazole-H), 7.86 (s, 1H), 7.60 (s, 2H, -NH2), 7.38 (dd, 1H, J =7.5, 4.02, Ar-H), 7.20-7.22 (m,2H, J =7.5, Ar-H), 2.20 (s, 3H, Ar-CH3); 13C NMR spectrum (100 MHz, DMSO-d6), δ, ppm: 172.2, 159.6, 143.2,138.9, 133.7, 132.5, 129.0, 128.0, 127.0, 120.7 and 18.3; MS (ESI) m/z 268.0 [M + H] + [11].
|
|
|