| Identification | Back Directory | [Name]
2-Bromo-6-methoxybenzothiazole | [CAS]
2941-58-4 | [Synonyms]
2-Bromo-6-methoxybenzothiazoL
2-BROMO-6-METHOXYBENZOTHIAZOLE
Benzothiazole, 2-bromo-6-methoxy-
2-bromo-6-methoxybenzo[d]thiazole | [Molecular Formula]
C8H6BrNOS | [MDL Number]
MFCD08459026 | [MOL File]
2941-58-4.mol | [Molecular Weight]
244.11 |
| Chemical Properties | Back Directory | [Boiling point ]
335.4±34.0 °C(Predicted) | [density ]
1.666±0.06 g/cm3(Predicted) | [storage temp. ]
Inert atmosphere,2-8°C | [pka]
0.53±0.10(Predicted) |
| Hazard Information | Back Directory | [Uses]
2-Bromo-6-methoxybenzothiazole is used as organic synthesis intermediate and pharmaceutical intermediate, mainly used in laboratory research and development process and chemical production process. | [Synthesis]
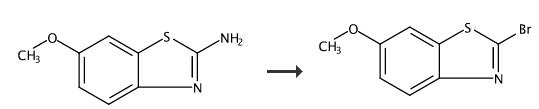
2-Bromo-6-methoxybenzo[d]thiazole (B2) (Scheme 1). 6- Methoxybenzo[d]thiazol-2-amine (5.0 g; 27.7 mmol) was dissolved in 125 mL CHBCN. t- Butylnitrite (3.4 mL; 28 mmol) was added slowly. CuBr (5.0 g; 34.9 mmol) was added portion-wise through a funnel. The reaction was monitored by HPLC. After 3 hours, ethyl acetate (500 mL) was added, and the mixture was filtered through celite. The organic layer was washed with brine twice (200 mL each). The organic layer was dried over MgSO4. After filtration, the solvent was removed. The residual was dissolved in 50 mL CH2Cl2, and 2 g of silica gel was added. After drying, the silica gel loaded on a silica gel cake in hexane. The product was eluted with 5% ethyl acetate and 95% hexane. A yellowish band was collected (1.5 L). The solvent was removed to afford the product. Yield: 0.81 g 12%. |
|
|