| Identification | More | [Name]
FMOC-Glycine | [CAS]
29022-11-5 | [Synonyms]
9-FLUORENYLMETHOXYCARBONYL-GLYCINE
F-FMOC-GLYCINE
FMOC-GLY
FMOC-GLYCINE
FMOC-GLY-OH
FMOC-L-GLY
FMOC-L-GLYCINE
FMOC-L-GLY-OH
N-(9-FLUORENYLMETHOXYCARBONYL)-GLYCINE
N-9-FLUORENYLMETHYLOXYCARBONYLGLYCINE
N-9-FMOC-L-GLYCINE
N-[(9H-FLUOREN-9-YLMETHOXY)CARBONYL]GLYCINE
N-ALPHA-(9-FLUORENYLMETHOXYCARBONYL)-GLYCINE
N-ALPHA-(9-FLUORENYLMETHYLOXYCARBONYL)-GLYCINE
N-ALPHA-FMOC-GLYCINE
N-FMOC-GLYCINE
N-FMOC-L-GLYCINE
npc14692
Glycine-1-13C-N-FMOC
Glycine-2-13C-N-FMOC | [EINECS(EC#)]
249-373-3 | [Molecular Formula]
C17H15NO4 | [MDL Number]
MFCD00037140 | [Molecular Weight]
297.31 | [MOL File]
29022-11-5.mol |
| Chemical Properties | Back Directory | [Appearance]
white to light yellow crystal powde | [Melting point ]
174-175 °C(lit.)
| [Boiling point ]
438.82°C (rough estimate) | [density ]
1.1671 (rough estimate) | [refractive index ]
1.4500 (estimate) | [storage temp. ]
2-8°C
| [solubility ]
almost transparency in Methanol | [form ]
Powder | [pka]
3.89±0.10(Predicted) | [color ]
White | [Detection Methods]
HPLC,NMR | [BRN ]
2163967 | [InChI]
InChI=1S/C17H15NO4/c19-16(20)9-18-17(21)22-10-15-13-7-3-1-5-11(13)12-6-2-4-8-14(12)15/h1-8,15H,9-10H2,(H,18,21)(H,19,20) | [InChIKey]
NDKDFTQNXLHCGO-UHFFFAOYSA-N | [SMILES]
C(O)(=O)CNC(OCC1C2=C(C=CC=C2)C2=C1C=CC=C2)=O | [CAS DataBase Reference]
29022-11-5(CAS DataBase Reference) |
| Safety Data | Back Directory | [Hazard Codes ]
Xi | [Risk Statements ]
R36/37/38:Irritating to eyes, respiratory system and skin . | [Safety Statements ]
S24/25:Avoid contact with skin and eyes .
S36:Wear suitable protective clothing .
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice . | [WGK Germany ]
3
| [HS Code ]
29242995 |
| Hazard Information | Back Directory | [Chemical Properties]
white to light yellow crystal powde | [Uses]
N-Fmoc-glycine is an N-Fmoc-protected form of Glycine (G615990). Glycine is a nonessential amino acid that acts as an inhibitory neyrotransmitter in the vertebrate central nervous system. Glycine also posesses cytoprotective against oxidant damage in the kidney. | [Preparation]
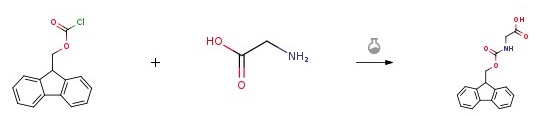
Glycine (Gly) (0.5 g, 6.66 mmol) was dissolved in 10% NaCO3 (14 ml) under stirring in a 50-ml flask. To the resulting solution, which had been put into an ice bath, 9-fluorenylmethoxycarbonyl chloride (Fmoc-Cl) (1.72 g, 6.66 mmol) in dioxane (12 ml) was gradually added. The reaction mixture was stirred at room temperature for 4 hours. Then added water (150 ml) to the mixture. The aqueous phase layer was separated from the reaction mixture and stripped with ether three times (75 ml×3). The stripped aqueous layer was acidified with 2N HCl aqueous solution to a pH=2, followed by extraction with ethyl acetate three times (75 ml×3). The organic phase layer was recovered and concentrated to obtain crude product of 1.70 g. The crude product was recrystallized in a mixed solvent of ethyl acetate hexane=1:2 (30 ml), and white solid denoted as Fmoc-Gly-OH was obtained after filtration at a reduced pressure.
 | [General Description]
The product number for this product was previously 04-12-1001.
To obtain a certificate of analysis (CoA) of a lot that begins with the letter “A”, please select the option in the right hand menu “Request a COA for Lot#s starting with A”. | [reaction suitability]
reaction type: Fmoc solid-phase peptide synthesis |
|
|