| Identification | More | [Name]
Oxcarbazepine | [CAS]
28721-07-5 | [Synonyms]
10,11-DIHDYRO-10-OXO-5H-DIBENZ[B,F]AZEPINE-5-CARBOXAMIDE
10,11-DIHYDRO-10-OXO-5H-DIBENZ[B,F]AZEPINE-5-CARBOXAMIDE
5H-DIBENZ[B,F]AZEPINE-5-CARBOXAMIDE, 10,11-DIHYDRO-10-OXO-
OXACARBAZEPINE
OXCARBAMAZEPINE
OXCARBAZEPINE
TRILEPTAL
f)azepine-5-carboxamide,10,11-dihydro-10-oxo-5h-dibenz(
gp47680
10,11-Dihdyro-10-oxo-5H-dibenz[b,f]azepine-5-carboxamide, Trileptal
OXCARBAZEPINE(SUBJECTTOPATENTFREE)
Ocarbazepine
10,11-Dihydro-10-oxo-5h-dibenz[b,f]azepine-5-carboxamide, Oxacarbazepine
10-Oxo-10,11-dihydro-5H-dibenzo[b,f]azepine-5-carboxamide
Oxycarbamazepine | [EINECS(EC#)]
249-188-8 | [Molecular Formula]
C15H12N2O2 | [MDL Number]
MFCD00865307 | [Molecular Weight]
252.27 | [MOL File]
28721-07-5.mol |
| Questions And Answer | Back Directory | [Overview]
Oxcarbazepine is structurally a derivative of carbamazepine; used for the treatment of partial seizures in epileptic children and adults[1]. Compared with carbamazepine, it contains an extra oxygen atom to the benzylcarboxamide group, reducing the impact on the liver of metabolizing the drug, and also preventing the serious forms of anemia occasionally associated with carbamazepine, further reducing potential side effects, it is thought to have the same mechanism as carbamazepine - sodium channel inhibition[1]. Oxcarbazepine was approved for use as an anticonvulsant in Denmark in 1990, Spain in 1993, Portugal in 1997, and eventually for all other EU countries in 1999. It was approved in the US in 2000[2].
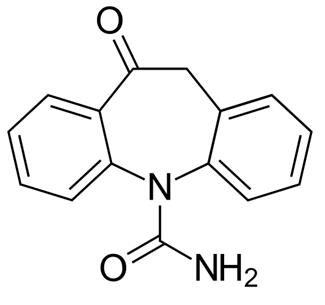
Figure 1 The chemical structure of Oxcarbazepine. ;
Oxcarbazepine is a second- generation AED supplied under the proprietary brand name of Trileptal® (Novartis, Basel) in the UK and USA. | [Physiochemical properties]
Oxcarbazepine is the 10-keto analogue of carbamazepine. It is a neutral lipophilic compound, and, like carbamazepine, very insoluble in water. It is not clear whether it is as unstable in humid conditions as is carbamazepine. The antiepileptic action of carbamazepine is, like carbamazepine[3–6], thought to be due primarily to blockage of voltage sensitive sodium channels, resulting in stabilization of hyper-excited neural membranes, inhibition of repetitive neuronal firing and inhibition of the spread of discharges. It also increases potassium conductance, reduces glutaminergic transmission and modulates calcium channel function. The rationale behind its original development was to use the parent molecule carbamazepine as a template, and to produce a drug as effective as carbamazepine, but with an improved side-effect profile. A key difference between the two drugs is that oxcarbazepine unlike carbamazepine is not metabolized to an epoxide derivative[7]. As the epoxide is responsible for some of the toxic effects of carbamazepine, the lack of epoxidation of Oxcarbazepine is probably one reason for its better side effect profile. Its biotransformation is largely by hydroxylation, to an active non-toxic 10-monohydroxy metabolite(MHD: 10,11 dihydro-10-hydroxy-5Hdibenzol[b,f]azepine-5-carboxamide)[7]. The pharmacological action of the drug is exerted primarily though this metabolite, and oxcarbazepine is in essence a prodrug of MHD.
| [Pharmacokinetics]
Oxcarbazepine is absorbed almost completely after oral ingestion, and this is an advantage over carbamazepine. Absorption is not affected by food[12]. Oxcarbazepine is rapidly and almost completely metabolized to the biologically active 10-monohydroxy metabolite MHD. Following ingestion, there is little parent drug circulating in the plasma, although MHD is widely distributed to brain and other lipid tissues[3,7-11]. The volume of distribution is 0.3–0.8 L/kg and it is 38% bound to plasma proteins. Foetal and maternal plasma concentrations of the drug are similar(as judged by the neonatal and maternal levels in one case) and the plasma: breast milk ratio of oxcarbazepine is 0.5.
Oxcarbazepine is rapidly and extensively metabolized in the liver via a reductive pathway, and less than 1% of the drug is excreted unchanged in the urine[3,7-11]. The primary metabolite MHD is conjugated to a glucuronide compound. It is not subject to epoxidation as is the case of carbamazepine. The biotransformation is rapid and almost complete, and only trace amounts of oxcarbazepine are found in the blood. A small amount of a dihydroxy derivative(DHD) is also formed. Peak serum concentrations of MHD are reached in 4–6 hours. The plasma half-life of MHD is about 8–10 hours, and is not altered by concomitant antiepileptic drug therapy. There is a linear relationship between dose and serum levels of the drug and its metabolite in the usual clinical dose ranges. The drug can be largely excreted by the kidneys, 83% as MHD or its glucuronide, 4–7% as the DHD, and 0.3–3% as oxcarbazepine. Due to this dependency on renal excretion, the dose of oxcarbazepine may need to be reduced in the presence of severe renal impairment[13].
| [Indication]
Oxcarbazepine is indicated for use as monotherapy or adjunctive therapy in the treatment of partial seizures in adults with epilepsy and as adjunctive therapy in the treatment of partial seizures in children ages 4-16 with epilepsy[1, 14].
Recommendations summarized from NICE (2012)
- Seizure types: first line (generalized tonic- clonic seizures, focal seizures), adjunctive (focal seizures), contraindicated (generalized tonic- clonic seizures if there are absence or myoclonic seizures, or if juvenile myoclonic epilepsy is suspected, tonic/ atonic seizures, absence seizures, myoclonic seizures).
- Epilepsy types: first line (epilepsy with generalized tonic- clonic seizures only, benign epilepsy with centrotemporal spikes, Panayiotopoulos syndrome, late- onset childhood occipital epilepsy), adjunctive (benign epilepsy with centrotemporal spikes, Panayiotopoulos syndrome, lateonset childhood occipital epilepsy), contraindicated (absence syndromes, juvenile myoclonic epilepsy, idiopathic generalized epilepsy, Dravet syndrome, Lennox– Gastaut syndrome).
| [Mode of action]
The exact mechanism that oxcarbazepine exerts its anticonvulsant effect is unknown. It is known that the pharmacological activity of oxcarbazepine occurs primarily through its 10-monohydroxy metabolite(MHD). In vitro studies indicate an MHD can lead to the blockade of voltage-sensitive sodium channels, resulting in stabilization of hyperexcited neuronal membranes, inhibition of repetitive neuronal discharges, and diminution of propagation of synaptic impulses.
| [Dose titration]
300 mg bd, increased by 300– 600 mg every 7 days; usual maintenance 600– 2400 mg daily, in divided doses.
| [Plasma levels monitoring]
Plasma level monitoring of oxcarbazepine is not routinely warranted. Although correlations between dosage and plasma levels of oxcarbazepine, and between plasma levels and clinical efficacy or tolerability are rather tenuous, monitoring of the plasma levels may be useful to rule out non- compliance or in patients with changes in renal function, patients with concomitant use of liver enzyme- inducing drugs and during pregnancy.
| [Interactions]
With AEDs
- Strong inducers of cytochrome P450 enzymes (i.e. carbamazepine, phenytoin, phenobarbital) have been shown to decrease the plasma levels of oxcarbazepine’s pharmacologically active metabolite.
- Oxcarbazepine and its pharmacologically active metabolite are weak inducers of the cytochrome P450 enzymes CYP3A4 and CYP3A5 responsible for the metabolism of a other AEDs (e.g. carbamazepine) resulting in a lower plasma concentration of these medicinal products.
- Concomitant therapy of oxcarbazepine and lamotrigine has been associated with an increased risk of adverse events (nausea, somnolence, dizziness, and headache).
With other drugs
- Oxcarbazepine and its pharmacologically active metabolite are weak inducers of the cytochrome P450 enzymes CYP3A4 and CYP3A5 responsible for the metabolism of a large number of drugs, for example, immunosuppressants (e.g. ciclosporin, tacrolimus) and oral contraceptives.
With alcohol/food
- Caution should be exercised if alcohol is taken in combination with oxcarbazepine, due to a possible additive sedative effect.
- There are no specific foods that must be excluded from diet when taking oxcarbazepine.
| [Adverse reactions]
The side-effect profile of oxcarbazepine is similar in nature to that of carbamazepine, although the frequency and severity of side effects have been shown to be less[3, 8, 11, 15, 16]. The commonest dose-related side effects are fatigue, headache, dizziness and ataxia. In a comparative monotherapy trial in 235 outpatients with newly diagnosed epilepsy, some side effects were reported by 68% receiving oxcarbazepine and 74% receiving carbamazepine, but the mean number of side-effects per patient was lower with oxcarbazepine compared with carbamazepine(2.8 vs. 3.5)[18]. Other side effects included nausea and gastrointestinal disturbance. Two studies have shown no impairment of cognitive function after 4–12 months of therapy with oxcarbazepine[17, 18]. In the comparative randomized controlled trials in both adults and children, the side-effect profile of the drug was better than with carbamazepine or phenytoin, and oxcarbazepine scored better on patients’ and physicians’ rating scales than carbamazepine, valproate or phenytoin[3, 8, 9, 11]. The withdrawal rates from side effects in the published monotherapy studies of oxcarbazepine can be take as a good index of tolerability. These were better on oxcarbazepine when compared to published studies of carbamazepine, valproate and phenytoin. There has been no published comparison of the tolerability of oxcarbazepine and newer antiepileptic drugs or slow-release carbamazepine, which would be of interest in view of the improved tolerability of the slow release compared to the standard carbamazepine formulation.
Skin rash is relatively common[16](up to 10% of all patients) and is the main reason for discontinuation of the drug in the comparative monotherapy studies[3, 8, 9, 11,16]. The rash is similar to that of carbamazepine, although cross-reactivity with carbamazepine is present in only about 25–30% of cases, and so oxcarbazepine is a useful drug in patients who are shown to have carbamazepine hypersensitivity1, 11. Oxcarbazepine, like carbamazepine, may cause hyponatraemia presumably due to an antidiuretic hormone-like effect[19, 20]. The effect seems to be greater with oxcarbazepine than carbamazepine, and about 20% of all patients show a serum sodium level below 135 mmol/l. The degree of hyponatraemia is however usually mild and is generally asymptomatic and not of clinical importance. It is a dose-dependent effect, and alleviated by dose reduction or water restriction.
| [Precautions]
If you will take oxcarbazepine, the following tips should be concerned[21]:
You should not take oxcarbazepine if you are allergic to oxcarbazepine or eslicarbazepine. You should also let your doctor know if you have the history of either one or several of the following cases: liver disease; kidney disease; mood problems or suicidal thoughts; or an allergy to carbamazepine(Carbatrol, Tegretol).
Some people have thoughts about suicide while taking oxcarbazepine. Your doctor will need to check your progress at regular visits. Your family or other caregivers should also be alert to changes in your mood or symptoms. You should be cautious regarding on starting or stopping taking oxcarbazepine during pregnancy. You should seek advice from your doctor. Having a seizure during pregnancy could harm both mother and baby. Tell your doctor right away if you become pregnant while taking oxcarbazepine for seizures. If you are pregnant, your name may be listed on a pregnancy registry to track the effects of oxcarbazepine on the baby. Oxcarbazepine can make birth control pills less effective. Ask your doctor about using a non-hormonal birth control(condom, diaphragm with spermicide) to prevent pregnancy. Breast-feed should not be allowed while you are taking oxcarbazepine. Do not give this medicine to a child without medical advice. There are specific age restrictions for the use of oxcarbazepine in children regarding the dose form and whether it is used alone or with other medicines.
| [Special populations]
Hepatic impairment
- Mild to moderate hepatic impairment does not affect the pharmacokinetics of oxcarbazepine and its active metabolite. Oxcarbazepine has not been studied in patients with severe hepatic impairment.
Renal impairment
- Dose adjustment (halve initial dose and increase according to response at intervals of at least 1 week) is recommended in patients with renal impairment and lower creatinine clearance.
Pregnancy
- Data on oxcarbazepine associated with congenital malformation are limited. There is no increase in the total rate of malformations with oxcarbazepine, compared with the rate observed in the general population. However, a moderate teratogenic risk cannot be completely excluded.
- If women receiving oxcarbazepine become pregnant or plan to become pregnant, the use of this drug should be carefully re- evaluated. Minimum effective doses should be given, and monotherapy whenever possible should be preferred at least during the first 3 months of pregnancy.
- During pregnancy, an effective antiepileptic oxcarbazepine treatment must not be interrupted, since the aggravation of the illness is detrimental to both the mother and the foetus.
- Oxcarbazepine and its active metabolite are excreted in human breastmilk. As the effects on the infant exposed to oxcarbazepine by this route are unknown, oxcarbazepine should not be used during breast-feeding.
| [Behavioural and cognitive effects in patients with epilepsy]
Similarly to carbamazepine, oxcarbazepine is generally considered to pose a low risk for adverse psychiatric effects (especially emotional lability, insomnia, abnormal thinking— usually occurring at high dosages) in patients with epilepsy. Moderate cognitive problems affecting attention and concentration have occasionally been reported (especially at high doses).
| [Psychiatric use]
Oxcarbazepine’s principal use in patients with psychiatric disorder is the treatment of bipolar disorder (mania), although there are no approved indications in psychiatry. Oxcarbazepine has been proposed as a potential option for add- on therapy in the treatment of bipolar disorder, although it remains to be determined whether oxcarbazepine is effective in the acute treatment of bipolar depression or the maintenance treatment of bipolar disorder. Preliminary evidence suggests that oxcarbazepine may exert beneficial effects on behavioural disorders, particularly impulsive aggression. There is also evidence for the potential usefulness of oxcarbazepine in obsessivecompulsive disorder and anxiety disorders (panic disorder, post- traumatic stress disorder). Other potential off- label uses are alcohol withdrawal and dependence, benzodiazepine withdrawal, and cocaine abuse/dependence.
| [References]
- https://www.drugbank.ca/drugs/DB00776
- https://www.pharma.us.novartis.com/product-list
- Tecoma, E. S. Oxcarbazepine Epilepsia 1999; 40[Suppl. 5]:S37–S46.
- Schutz, M., Brugger, F., Gentsch, C., McLean, M. and Olpe, M. Oxcarbazepine: preclinical profile and putative mechanism of action. Epilepsia 1994; 35[Suppl. 5]: S5–S9.
- Kubova, H. et al. Anticonvulsant action of oxcarbazepine, hydroxycarbamazepine and carbamazepine against metrazolinduced seizures in developing rats. Epilepsia 1993; 34: 188–192.
- Schmurz, M. et al. Oxcarbazepine: preclinical anticonvulsant profile and putative mechanisms of action. Epilepsia 1994; 35[Suppl. 5]: S47–S50.
- Schutz, H., Feldmann, K. F., Faigle, J. W., Kriemler, H. and Winkler, T. The metabolism of 14C-oxcarbazepine in man. Xenobiotica 1986; 19: 769–778.
- Schachter, S. C. Oxcarbazepine: current status and clinical applications. Experimental Opinions of Investigative Drugs 1999; 8: 1–10.
- Dam, M. and Østergaard, L. H. Antiepileptic Drugs. 4th Edition.[Eds R. H. Levy, R. H. Manson and B. S. Meldrum]. New York, Raven Press, 1995: pp. 987–995.
- Lloyd, P., Flesch, G. and Dielerle, W. Clinical pharmacology and pharmacokinetics of oxcarbazepine. Epilepsy 1994; 35[Suppl. 3]: S10–S13.
- Gram, L. Oxcarbazepine. Epilepsy: A Comprehensive Textbook[Eds J. Engel and T. A. Pedley]. Philadelphia, PA, Lippencott Raven, 1997: p. 15416.
- 12. Degen, P. H., Flesch, G., Cardot, J. M. et al. The influence of food on the disposition of the antiepileptic oxcarbazepine and its major metabolites in healthy volunteers. Biopharmaceutics and Drug Disposition 1994; 15: 519–526.
- 13. Rouan, M. C., Lecaillon, M. B., Godbillon, J. et al. The effects of renal impairment on the pharmacokinetics of oxcarbazepine and its metabolites. European Journal of Clinical Pharmacology 1994; 47: 161–167.
- 14. https://www.rxlist.com/trileptal-drug.htm#indications
- 15. Bill, P. A., Vigonius, U., Pohlmann, A. et al. A double-blind, controlled clinical trial of oxcarbazepine versus phenytoin in adults with previously untreated epilepsy. Epilepsy Research 1997; 27: 195–204.
- 16. Dam, M., Ekberg, R., Loyning, Y. et al. A double-blind study comparing oxcarbazepine and carbamazepine in patients with newly diagnosed, previously untreated epilepsy. Epilepsy Research 1989; 3: 70–76.
- 17. A¨ ikia¨,M., Ka¨lvia¨inen, R., Sivenius, J., Halonen, T. and Riekkinen, P. J. Cognitive effects of oxcarbazepine and phenytoin monotherapy in newly diagnosed epilepsy: one year follow-up. Epilepsy Research 1992; 1: 199–203.
- 18. Curran, H. V. and Java, R. Memory and psychomotor effects of oxcarbazepine in healthy human volunteers. European Journal of Clinical Pharmacology 1993; 44: 529–533.
- 19. Nielsen, O. A. et al. Oxcarbazepine-induced hyponatraemia, a cross sectional study. Epilepsy Research 1988; 2: 269–271.
- 20. Pendlebury, S. C. et al. Hyponatraemia during oxcarbazepine therapy. Human Toxicology 1989; 8: 337–344.
- https://www.drugs.com/mtm/oxcarbazepine.html
|
| Hazard Information | Back Directory | [Description]
Oxcarbazepine is a new antiepileptic carbamazepine derivative, reportedly better
tolerated than carbamazepine. It appears to be most effective in partial epilepsy with
complex seizures. | [Chemical Properties]
Pale Yellow Powder | [Originator]
Ciba-Geigy (Switzerland) | [Uses]
A metabolite of Eslicarbazepine acetate, (BIA 2-093), a novel central nervous system drug. | [Uses]
beta-adrenergic blocker | [Uses]
The keto derivative of Carbamazepine. Used as an anticonvulsant | [Definition]
ChEBI: A dibenzoazepine derivative, having a carbamoyl group at the ring nitrogen, substituted with an oxo group at C-4 of the azepeine ring which is also hydrogenated at C-4 and C-5. It is a anticholinergic anticonvulsant and mood stabilizing drug, used primaril
in the treatment of epilepsy. | [Brand name]
Trileptal (Novartis). | [Biological Functions]
Oxcarbazepine is chemically and pharmacologically
closely related to carbamazepine, but it has much less
capacity to induce drug-metabolizing enzymes. This
property decreases the problems associated with drug
interactions when oxcarbazepine is used in combination
with other drugs. The clinical uses and adverse effect
profile of oxcarbazepine appear to be similar to those of
carbamazepine. | [General Description]
Oxcarbazepine, marketed under the trade name Trileptal?, is an anticonvulsant developed and prescribed for treatment of epilepsy. In recent years, Oxcarbazepine has shown efficacy in treatment of mood disorders. This certified solution standard is suitable as starting material for the preparation of calibrators and controls in oxcarbazepine testing by GC/MS or LC-MS/MS. | [Biological Activity]
Anticonvulsant; protects mice and rats against generalized tonic-clonic seizures induced by electroshock. Thought to act via inhibition of sodium channel activity. | [Biochem/physiol Actions]
Anticonvulsant, antineuralgic. Inhibits veratrine-induced transmitter release. | [Mechanism of action]
Although oxcarbazepine is less potent that CBZ, its mechanism of action is similar. The majority of the pharmacological
activity for oxcarbazepine is attributed to its primary metabolite, 10-monohydroxycarbazepine (MHD), the plasma
levels of which may be ninefold higher than those for CBZ. Both oxcarbazepine and MHD produce a blockade of voltage�dependent sodium channels, thus decreasing repetitive firing and spread of electrical activity. An additional action on calcium and potassium channels may contribute to the therapeutic effect. Like carbamazepine, oxcarbazepine may worsen juvenile
myoclonic or absence seizures. | [Clinical Use]
Oxcarbazepine (Trileptal?) is the 10-keto analogue of carbamazepine. It is indicated as monotherapy or adjunctive
therapy for partial seizures in adults with epilepsy, as monotherapy for the treatment of partial seizures in children 4 years of
age or older, and as adjunct therapy in children 2 to 4 years of age. | [Side effects]
Patients with hypersensitivity reactions to carbamazepine can be expected to show cross-sensitivity (e.g., rash) or related
problems to oxcarbazepine. The improved toxicity profile for oxcarbazepine when compared to CBZ may result from absence of
the epoxide or CBZ-iminoquinone metabolites. The most common side effects are headache, dizziness, nystagmus, blurred
vision, somnolence, nausea, ataxia, and fatigue. The incidence of adverse effects has been related to elevated serum MHD
concentrations. Adverse effects on cognitive status, hyponatremia, and serious dermatological reactions have been
reported, as has hyponatremia. | [Drug interactions]
Potentially hazardous interactions with other drugs
Antidepressants: antagonism of anticonvulsant
effect; avoid with St John’s wort.
Antiepileptics: concentration of perampanel reduced,
also increased oxcarbazepine concentration.
Antimalarials: anticonvulsant effect antagonised by
mefloquine.
Antipsychotics: antagonism of anticonvulsant effect.
Antivirals: concentration of rilpivirine and possibly
daclatasvir and simeprevir reduced - avoid; possibly
reduces dolutegravir concentration.
Ciclosporin: metabolism accelerated (reduced
ciclosporin concentration).
Clopidogrel: possibly reduced antiplatelet effect.
Cytotoxics: concentration of imatinib reduced -
avoid.
Guanfacine: possibly reduces guanfacine
concentration - increase dose of guanfacine.
Oestrogens and progestogens: metabolism
accelerated (reduced contraceptive effect).
Orlistat: possible increased risk of convulsions.
Tacrolimus: metabolism accelerated (reduced
tacrolimus concentration).
Ulipristal: possibly reduces contraceptive effect. | [Metabolism]
Oxcarbazepine is rapidly reduced by cytosolic enzymes
in the liver to the active monohydroxy metabolite
(licarbezine, or MHD). MHD is metabolised further by
conjugation with glucuronic acid.
Minor amounts (4% of the dose) are oxidised to a
pharmacologically inactive metabolite. Oxcarbazepine is
excreted in the urine mainly as metabolites. | [storage]
Store at RT |
|
|