| Identification | Back Directory | [Name]
SB216763 | [CAS]
280744-09-4 | [Synonyms]
SB 216763
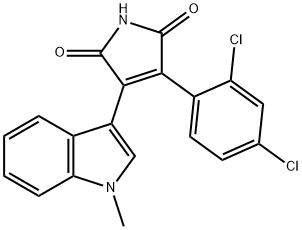
3-(2,4-Dichlorophenyl)-4-...
3-(2,4-DICHLOROPHENYL)-4-(1-METHYL-1H-INDOL-3-YL)-1H-PYRROLE-2,5-DIONE
3-(2,4-dichlorophenyl)-4-(1-methyl-1H-indol-3-yl)-2,5-dihydro-1H-pyrrole-2,5-dione | [Molecular Formula]
C19H12Cl2N2O2 | [MDL Number]
MFCD03846748 | [MOL File]
280744-09-4.mol | [Molecular Weight]
371.217 |
| Chemical Properties | Back Directory | [Melting point ]
287-288.6 °C(lit.)
| [Boiling point ]
598.1±50.0 °C(Predicted) | [density ]
1.47±0.1 g/cm3(Predicted) | [storage temp. ]
−20°C
| [solubility ]
DMSO: 20 mg/mL, soluble
| [form ]
Orange solid | [pka]
7.27±0.60(Predicted) | [color ]
orange
| [Stability:]
Stable for 2 years from date of purchase as supplied. Solutions in DMSO may be stored at -20° for up to 3 months. |
| Hazard Information | Back Directory | [Biological Activity]
Potent and selective glycogen synthase kinase-3 (GSK-3) inhibitor (K i = 9 nM for GSK-3 α ); competes with ATP. Has minimal activity against 24 other protein kinases (IC 50 > 10 μ M). Stimulates glycogen synthesis, gene transcription and is neuroprotective. | [Description]
SB-216763 (280744-09-4) is a potent and selective inhibitor of glycogen synthase kinase-1 (GSK-3) IC50=34.3 nM.1 Acts at the ATP-binding domain. Displays protective effects in lung fibrosis mouse model.2 Displays neuroprotective effects on cultured neurons.3 SB-216763 maintains mouse embryonic stem cells in a pluripotent state.4?Cell permeable. | [Uses]
SB 216763 is a potent and selective cell permeale ATP-competitive inhibitor of GSK3a. It stimulates glycogen synthesis in Chang human liver cells. | [Definition]
ChEBI: 3-(2,4-dichlorophenyl)-4-(1-methyl-3-indolyl)pyrrole-2,5-dione is a member of indoles and a member of maleimides. | [Biochem/physiol Actions]
SB-216763 is a small molecule that competes with ATP and potently inhibits the activity α and β isozymes of GSK-3. It acts as neuroprotectant and prevents neuronal cell death induced by PI3-kinase pathway. It also delays preconditioning, reduces infarct size and prevents cardiac ischemia. | [storage]
Room temperature | [References]
1) Coghlan et al. (2000), Selective small molecule inhibitors of glycogen synthase kinase-3 modulate glycogen metabolism and gene transcription; Chem. Biol., 7 793
2) Gurrieri et al. (2010), 3-(2,4-dichlorophenyl)-4-(1-methyl-1H-indol-3-yl)-1H-pyrrole-2,5-dione (SB216763), a glycogen synthase kinase-3 inhibitor, displays therapeutic properties in a mouse model of pulmonary inflammation and fibrosis; J. Pharmacol. Exp. Ther., 332 785
3) Cross et al. (2001), Selective small-molecule inhibitors of glycogen synthase kinase-3 activity protect primary neurons from death; J. Neurochem., 77 94
4) Kirby et al. (2012), Glycogen synthase kinase 3 (GSK3) inhibitor , SB-216763, promotes pluripotency in mouse embryonic stem cells; PLoS One, 7 e39329 |
|
|