| Identification | Back Directory | [Name]
AZOXYMETHANE | [CAS]
25843-45-2 | [Synonyms]
AOM
azoxy-methan
AZOXYMETHANE
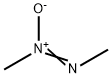
CH3N=N(→O)CH3
Methane, azoxy-
ONN-Azoxydimethane
Dimethyldiazene 1-oxide
Azoxymethane
DISCONTINUED
1,2-Dimethyldiazene 1-oxide
(Z)-1,2-diMethyldiazene oxide
"N,N-Dimethyldiazene N-oxide"
[(E)-methyl-NNO-azoxy]methane
(Z)-1,2-Dimethyldiazene 1-oxide
(E)-dimethyldiazen-1-ium-1-olate
Diazene, dimethyl-,1-oxide (9CI)
AZOXYMETHANE, PRACTICAL GRADE, 13.4M | [Molecular Formula]
C2H6N2O | [MDL Number]
MFCD00126912 | [MOL File]
25843-45-2.mol | [Molecular Weight]
74.08 |
| Chemical Properties | Back Directory | [Boiling point ]
97-99 °C(lit.)
| [density ]
0.991 g/mL at 25 °C(lit.)
| [refractive index ]
1.4368 (estimate) | [Fp ]
24 °C | [storage temp. ]
−20°C
| [solubility ]
Soluble in water, ethanol, and ether | [form ]
Liquid | [color ]
Oil | [Specific Gravity]
0.991 | [InChI]
InChI=1S/C2H6N2O/c1-3-4(2)5/h1-2H3 | [InChIKey]
DGAKHGXRMXWHBX-UHFFFAOYSA-N | [SMILES]
[N+]([O-])(C)=NC | [EPA Substance Registry System]
Azoxymethane (25843-45-2) |
| Safety Data | Back Directory | [Hazard Codes ]
T | [Risk Statements ]
45-46-10-25-34-36/38 | [Safety Statements ]
53-26-36/37/39-45 | [RIDADR ]
UN 1992 3/PG 3
| [WGK Germany ]
3
| [RTECS ]
PA2975000 | [Safety Profile]
Suspected carcinogen
with experimental carcinogenic and
mmorigenic data. Poison by subcutaneous
route. An experimental teratogen. Mutation
data reported. When heated to
decomposition it emits toxic fumes of Nox | [Toxicity]
mma-sat 13,600 mmol/L/20M CNREA8 38,4585,78 |
| Hazard Information | Back Directory | [General Description]
Clear oily liquid. | [Air & Water Reactions]
AZOXYMETHANE is sensitive to prolonged exposure to air and elevated temperatures . Soluble in water. | [Reactivity Profile]
AZOXYMETHANE is an azo compound. Azo, diazo, azido compounds can detonate. This applies in particular to organic azides that have been sensitized by the addition of metal salts or strong acids. Toxic gases are formed by mixing materials of this class with acids, aldehydes, amides, carbamates, cyanides, inorganic fluorides, halogenated organics, isocyanates, ketones, metals, nitrides, peroxides, phenols, epoxides, acyl halides, and strong oxidizing or reducing agents. Flammable gases are formed by mixing materials in this group with alkali metals. Explosive combination can occur with strong oxidizing agents, metal salts, peroxides, and sulfides. | [Health Hazard]
ACUTE/CHRONIC HAZARDS: AZOXYMETHANE is highly flammable and sensitive to elevated temperatures. Upon decomposition it emits toxic fumes. | [Fire Hazard]
Flash point data for AZOXYMETHANE are not available. AZOXYMETHANE is probably highly flammable. | [Uses]
Azoxymethane (AOM), a gene mutation agent, may be used with dextran sulfate sodium (DSS) to create cancer models in laboratory animals which can be used to study mechanisms of cancer progression and chemoprevention. | [Biochem/physiol Actions]
Carcinogen that induces O6-methylguanine adducts in DNA leading to G→A transitions. Induces tumorigenesis in the colon of laboratory animals and is used to study the mechanism of cancer progression and chemoprevention. |
|
| Company Name: |
Sigma-Aldrich
|
| Tel: |
021-61415566 800-8193336 |
| Website: |
https://www.sigmaaldrich.cn |
|