| Identification | Back Directory | [Name]
Dibutoxymethane | [CAS]
2568-90-3 | [Synonyms]
Butylal
Dibutyl formal
Dibutoxymethane
Di-n-butyl formal
5,7-Dioxaundecane
5,7-Dioxahendecane
DI-N-BUTOXYMETHANE
Methane, dibutoxy-
Dibutoxymethane>
Bis(butyloxy)methane
1-butoxymethoxy-butane
1-(Butoxymethoxy)butane
formaldehydedibutylacetal
Formaldehyde dibutyl acetal
FORMALDEHYDE DI-N-BUTYL ACETAL
1,1'-[methylenebis(oxy)]dibutane
Butane, 1,1-methylenebis(oxy)bis-
1,1’-[methylenebis(oxy)]bis-butan
1,1’-[methylenebis(oxy)]bis-Butane
FORMALDEHYDE DI-N-BUTYL ACETAL 98+%
(2-methylpropoxy)methoxy-2-methylpropane
Formaldehyde Dibutyl Acetal
5,7-Dioxahendecane
Formaldehyde dibutyl acetal puriss., >=98.5% (GC) | [EINECS(EC#)]
219-909-0 | [Molecular Formula]
C9H20O2 | [MDL Number]
MFCD00059270 | [MOL File]
2568-90-3.mol | [Molecular Weight]
160.25 |
| Hazard Information | Back Directory | [Uses]
Formaldehyde dibutyl acetal is a halogen-free and less toxic solvent that can be used to solubilize commercial low-density polyethylene (LDPE) samples to analyze molecular weight distribution using gel permeation chromatography (GPC). It can also be used as a reactant to prepare butoxymethyltriphenylphosphonium iodide, which is used for carbon homologation and also as a useful key intermediate in organic synthesis. | [Synthesis Reference(s)]
The Journal of Organic Chemistry, 45, p. 3341, 1980 DOI: 10.1021/jo01304a039 | [General Description]
Formaldehyde dibutyl acetal is an acetal used in the manufacture of synthetic resins, antiseptics, deodorants, and fungicides. It is also used as a fuel additive to increase the octane number of gasoline or the n-cetane number of diesel fuels and reduce smoke and particulate emissions. |
| Questions And Answer(Q&A) | Back Directory | [Preparation]
A flask containing 15 gm (0.5 mole) of paraformaldehyde, 74 gm (1.0 mole) of η-butyl alcohol, and 2.0 gm of anhydrous ferric chloride is refluxed for 10 hr. The lower layer of 3-4 ml of material is discarded and then 50 ml of 10% aqueous sodium carbonate solution is added to remove the ferric chloride as ferric hydroxide. The product is shaken with a mixture of 40 ml of 20% hydrogen peroxide and 5 ml of 10% sodium carbonate solution at 45°C in order to remove any remaining aldehyde. The product is also washed with water, dried, and distilled from excess sodium metal to afford 62 gm (78%).
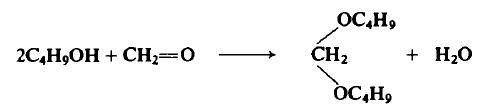 |
|
|