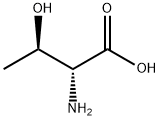
| Identification | More | [Name]
D(-)-allo-Threonine | [CAS]
24830-94-2 | [Synonyms]
(2R,3R)-(-)-2-AMINO-3-HYDROXYBUTYRIC ACID
(2R,3R)-2-AMINO-3-HYDROXYBUTYRIC ACID
(2R,3R)-(-)-ALLO-THREONINE
D(-)-ALLO-THREONINE
D-ALLO-THREONINE
H-D-ALLO-THR-OH
(r)-allothreonine
Allothreonine, D-
d-allothreonin
(2R,3S)-2-Amino-3-hydroxybutyric acid~H-D-Thr-OH
(2S,3S)-2-amino-3-hydroxy-butanoic acid
(2R,3R)-2-Amino-3-hydroxybutanoic acid | [EINECS(EC#)]
246-488-0 | [Molecular Formula]
C4H9NO3 | [MDL Number]
MFCD00004526 | [Molecular Weight]
119.12 | [MOL File]
24830-94-2.mol |
| Chemical Properties | Back Directory | [Appearance]
white crystalline powder | [Melting point ]
276 °C (dec.)(lit.) | [alpha ]
-33.5 º (c=1, 1N HCl 24 ºC) | [Boiling point ]
222.38°C (rough estimate) | [density ]
1.3126 (rough estimate) | [refractive index ]
-10 ° (C=5, H2O) | [storage temp. ]
Store at RT. | [solubility ]
Water (Slightly) | [form ]
Solid | [pka]
2.19±0.10(Predicted) | [color ]
White to Off-White | [Water Solubility ]
soluble | [BRN ]
1721644 | [InChIKey]
AYFVYJQAPQTCCC-PWNYCUMCSA-N | [CAS DataBase Reference]
24830-94-2(CAS DataBase Reference) | [NIST Chemistry Reference]
D-allothreonine(24830-94-2) |
| Safety Data | Back Directory | [Safety Statements ]
S24/25:Avoid contact with skin and eyes . | [WGK Germany ]
3
| [RTECS ]
BA4050000
| [HS Code ]
29225090 | [Toxicity]
rat,LD50,intraperitoneal,6910mg/kg (6910mg/kg),BEHAVIORAL: MUSCLE CONTRACTION OR SPASTICITY)LUNGS, THORAX, OR RESPIRATION: DYSPNEA,Archives of Biochemistry and Biophysics. Vol. 64, Pg. 319, 1956. |
| Questions And Answer | Back Directory | [Description]
D(-)-allo-Threonine is an isomer of threonine. It is found as a constituent of an increasing group of biologically active peptides. Stereocontrolled syntheses of chiral and racemic key intermediates to thienamycin can be achieved from d-allo-threonine and trans-crotonic acid.
| [References]
https://www.alfa.com/en/catalog/H27050/
http://www.sigmaaldrich.com/catalog/product/sigma/t9643?lang=en®ion=US
|
| Hazard Information | Back Directory | [Chemical Properties]
white crystalline powder | [Uses]
They are found as constituents of an increasing group of biologically active peptides. Stereocontrolled syntheses of chiral and racemic key intermediates to thienamycin from d-allo-threonine and trans-crotonic acid. | [Definition]
ChEBI: D-allothreonine is the D-enantiomer of allothreonine. It occurs as a component of peptido-lipids in certain bacteria. It has a role as a bacterial metabolite. It is an allothreonine and a D-alpha-amino acid. It is an enantiomer of a L-allothreonine. It is a tautomer of a D-allothreonine zwitterion. | [Purification Methods]
Recrystallise D-allothreonine from aqueous EtOH or 50% EtOH. [Elliot J Chem Soc 62 1950, Birnbaum et al. J Biol Chem 194 455 1952, IR: Greenstein & Winitz The Chemistry of the Amino Acids J. Wiley, Vol 3 1961, Beilstein 4 IV 3170.] |
|
|