| Identification | More | [Name]
5-CHLOROTHIOPHENE-2-CARBOXYLIC ACID | [CAS]
24065-33-6 | [Synonyms]
5-CHLORO-2-THIOPHENECARBOXYLIC ACID
5-CHLOROTHIOPHENE-2-CARBOXYLIC ACID
AKOS B000092
RARECHEM AL BE 0303
TIMTEC-BB SBB003937
2-Thiophenecarboxylic acid, 5-chloro-
5-chloro-2-thiophenecarboxylicaci
5-Chlorothiophene-2-carboxylicacid,98%
2-CHLOROTHIOPHENE-5-FORMIC ACID
CHLOROTHIOPHENEDICARBOXYLICACID
5-CHLOROTHIOPHENE-2-CARBOXYLIC ACID 98% | [EINECS(EC#)]
-0 | [Molecular Formula]
C5H3ClO2S | [MDL Number]
MFCD00041426 | [Molecular Weight]
162.59 | [MOL File]
24065-33-6.mol |
| Chemical Properties | Back Directory | [Appearance]
White to light yellow crystal powder | [Melting point ]
154-158 °C(lit.)
| [Boiling point ]
287.0±20.0 °C(Predicted) | [density ]
1.466 (estimate) | [storage temp. ]
Keep in dark place,Sealed in dry,Room Temperature | [solubility ]
DMSO (Slightly), Methanol (Slightly) | [form ]
Powder | [pka]
3.32±0.10(Predicted) | [color ]
Cream-yellow | [Detection Methods]
HPLC | [BRN ]
118361 | [InChI]
InChI=1S/C5H3ClO2S/c6-4-2-1-3(9-4)5(7)8/h1-2H,(H,7,8) | [InChIKey]
QZLSBOVWPHXCLT-UHFFFAOYSA-N | [SMILES]
C1(C(O)=O)SC(Cl)=CC=1 | [CAS DataBase Reference]
24065-33-6(CAS DataBase Reference) | [NIST Chemistry Reference]
5-Chloro-2-thiophenecarboxylic acid(24065-33-6) |
| Safety Data | Back Directory | [Hazard Codes ]
Xi | [Risk Statements ]
R36:Irritating to the eyes.
R43:May cause sensitization by skin contact.
R36/37/38:Irritating to eyes, respiratory system and skin . | [Safety Statements ]
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice .
S36:Wear suitable protective clothing .
S37/39:Wear suitable gloves and eye/face protection . | [WGK Germany ]
3
| [RTECS ]
XM8400000
| [Hazard Note ]
Irritant | [HazardClass ]
IRRITANT | [HS Code ]
29349900 |
| Hazard Information | Back Directory | [Description]
5-Chlorothiophene-2-carboxylic acid is a biochemical reagent that can be used as a biological material or organic compound for life science-related research. It is also used as a ligand to synthesise the New Mono and Binuclear Ruthenium(II) Complexes[1].
| [Chemical Properties]
White to light yellow crystal powder | [Uses]
A metabolite of Rivaroxaban (R538000). | [Synthesis]
The compound 5-chlorothiophene-2-carboxylic acid is a crucial intermediate in the synthesis of rivaroxaban. Due to its significance, numerous researchers have investigated various methods of synthesizing it. The synthesis of this compound involves the utilization of different starting materials, namely, 2-chlorothiophene, 2-chloro-5-bromothiophene, and 5-chloro-2-acetylthiophene, depending on the specific approach employed.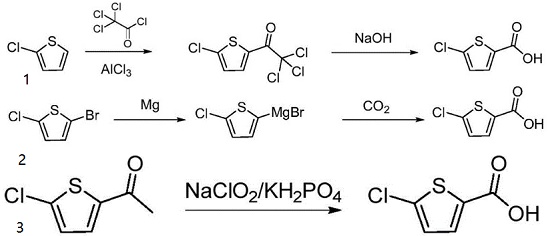
1. This method takes 2-chlorothiophene as an initial raw material, carrying out Friedel-crafts acylation with trichloroacetyl chloride under the action of aluminum trichloride to generate 2-trichloroacetyl-5-chlorothiophene, and then carrying out liquid alkali hydrolysis to obtain a target product.
2. This method reacts 5-chloro-2-bromothiophene with magnesium to generate a Grignard reagent, introducing carbon dioxide for inserting carbonyl and performing acid-base treatment to obtain a product.
3. 5-chloro-2-acetylthiophene is used as a raw material and is oxidized by sodium chlorite and potassium dihydrogen phosphate system to obtain a target compound. | [References]
[1] Kalaiyar Swarnalatha, Ramasamy Subramanian, Subramaniam Kamalesu . “Mono and binuclear ruthenium(II) complexes containing 5-chlorothiophene-2-carboxylic acid ligands: Spectroscopic analysis and computational studies.” Journal of Molecular Structure 1123 (2016): Pages 416-425.
|
|
|