| Identification | Back Directory | [Name]
2-DI-T-BUTYLPHOSPHINO-2'-(N,N-DIMETHYLAMINO)BIPHENYL | [CAS]
224311-49-3 | [Synonyms]
tBuDavePhos
98% tBuDavePhos
t-Butyl DavePhos
t-BuDavePhos 97%
2-(Di-tert-butylphosphino)-2'-
2-DI-T-BUTYLPHOSHINO-2''-(N,N-DIMETHYLAMINO)BIPHENYL
2-DI-T-BUTYLPHOSPHINO-2'-(N,N-DIMETHYLAMINO)BIPHENYL
2-DI-TERT-BUTYLPHOSHINO-2'-(N,N-DIMETHYLAMINO)BIPHENYL
2-DI-TERT-BUTYLPHOSPHINO-2'-(N,N-DIMETHYLAMINO)BIPHENYL
2-Di-t-butylphosphino-2'-(N,N-dimethylamino)biphenyl,98%
2′-(Di-tert-butylphosphino)-N,N-dimethylbiphenyl-2-amine
2-[2-(di-tert-butylphosphanyl)phenyl]-N,N-diMethylaniline
2-(Di-tert-butylphosphiNA)-2'-(N,N-diMethylaMiNA)biphenyl
2-Di-tert-butylphosphino-2'-(N,N-dimethylamino)biphenyl,98%
[2'-(DI-TERT-BUTYL-PHOSPHANYL)-BIPHENYL-2-YL]-DIMETHYL-AMINE
2'-(Di-tert-butylphosphino)-N,N-diMethyl-[1,1'-biphenyl]-2-aMine
2-Di-t-butylphosphino-2'-(N,N-dimethylamino))-1,1'-biphenyl, 98%
2-Di-t-butylphosphino-2'-(N,N-dimethylamino)biphenyl, (tBuDavePhos)
2-Di-t-butylphosphino-2'-(N,N-diMethylaMino)biphenyl,98% tBuDavePhos
2'-[Bis(1,1-dimethylethyl)phosphino]-N,N-dimethyl-[1,1'-biphenyl]-2-amine
2-Di-t-butylphosphino-2'-(N,N-dimethylamino))-1,1'-biphenyl, 98% tBuDavePhos
[1,1''-BIPHENYL]-2-AMINE, 2''-[BIS(1,1-DIMETHYLETHYL)PHOSPHINO]-N,N-DIMETHYL- | [EINECS(EC#)]
607-073-3 | [Molecular Formula]
C22H32NP | [MDL Number]
MFCD03426986 | [MOL File]
224311-49-3.mol | [Molecular Weight]
341.47 |
| Chemical Properties | Back Directory | [Appearance]
White crystals or crystalline powder | [Melting point ]
115-117 °C
| [Boiling point ]
451.6±38.0 °C(Predicted) | [storage temp. ]
Inert atmosphere,Room Temperature | [form ]
crystal | [pka]
5.07±0.18(Predicted) | [color ]
white | [Sensitive ]
Air Sensitive | [InChI]
InChI=1S/C22H32NP/c1-21(2,3)24(22(4,5)6)20-16-12-10-14-18(20)17-13-9-11-15-19(17)23(7)8/h9-16H,1-8H3 | [InChIKey]
PHLPNEHPCYZBNZ-UHFFFAOYSA-N | [SMILES]
C1(C2=CC=CC=C2P(C(C)(C)C)C(C)(C)C)=CC=CC=C1N(C)C |
| Questions And Answer | Back Directory | [Reaction]
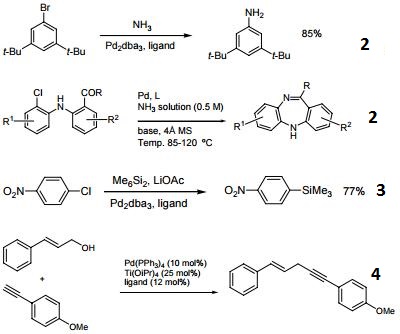
- Useful ligand for Pd-catalyzed carbon-oxygen bond forming reactions.
- Ligand used selective Pd-catalyzed arylation of ammonia. Application to the synthesis of dibenzodiazepines.
- Ligand used for selective Pd-catalyzed silylation of aryl chlorides.
- Ligand used for Pd(0)-catalyzed direct dehydrative coupling of terminal alkynes with allylic alcohols to access 1,4-enynes.
|
|
|