| Identification | Back Directory | [Name]
Fluoromethyl phenyl sulfone | [CAS]
20808-12-2 | [Synonyms]
FLUOROMETHYL PHENYL SULFONE
FLUOROMETHYLSULFONYLBENZENE
FluoromethylPhenylSulfone>
Phenyl Fluoromethyl Sulfone
Fluoromethanesulfonyl-benzene
Benzene, [(fluoromethyl)sulfonyl]- | [Molecular Formula]
C7H7FO2S | [MDL Number]
MFCD00191650 | [MOL File]
20808-12-2.mol | [Molecular Weight]
174.19 |
| Chemical Properties | Back Directory | [Melting point ]
53 °C | [Boiling point ]
151 °C(Press: 0.3 Torr) | [density ]
1.274±0.06 g/cm3(Predicted) | [storage temp. ]
under inert gas (nitrogen or Argon) at 2-8°C | [solubility ]
soluble in Methanol | [form ]
powder to crystal | [color ]
White to Almost white |
| Hazard Information | Back Directory | [Description]
Fluoromethyl phenyl sulfone is a useful nucleophilic monofluoromethylation reagent for the
synthesis of fluoromethyl alcohols and amines. In the nucleophilic reaction step, strong bases such
as LiHMDS and n-BuLi are used to generate the nucleophilic (phenylsulfonyl)fluoromethyl anion.
In the desulfonylation step, sodium/mercury amalgam and magnesium are the commonly used
reductive reagents. Besides, the addition reaction between fluoromethyl phenyl sulfone and
carbonyls can be used to prepare monofluoroaklenes via acylation–elimination. | [Uses]
Fluoromethyl phenyl sulfone can be used to prepare monofluoroaklenes and chiral α-Monofluoromethyl Amines. Highly stereoselective nucleophilic monofluoromethylation of (R)-(tert-butanesulfinyl)imines with fluoromethyl phenyl sulfone was achieved to afford α-monofluoromethylamines with a nonchelation-controlled stereoselectivity mode. By using the same chemistry, (R)-(tert-butanesulfinyl)imines bearing a terminal tosylate (OTs) group can be converted to α-monofluoromethylated cyclic secondary amines with high stereoselectivity. | [Reactions]
(1) Monofluoromethylation of aldehydes and ketones.
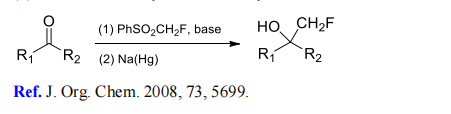
(2) Monofluoromethylation of aldimines and ketimines.
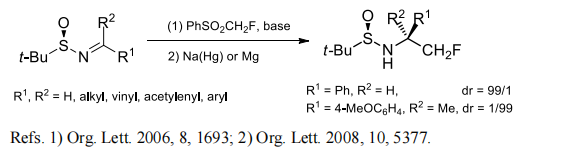
(3) Monofluoromethylenation of aldehydes and ketones.
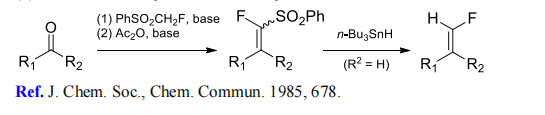
(4) (Phenylsulfonyl)fluoromethylation of esters.
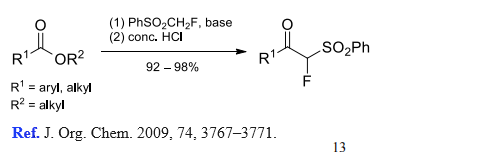
| [References]
[1] YA LI. Stereoselective Nucleophilic Monofluoromethylation of N-(tert-Butanesulfinyl)imines with Fluoromethyl Phenyl Sulfone[J]. Organic Letters, 2006. DOI:10.1021/ol060322t.
[2] M. INBASEKARAN. ChemInform Abstract: A NOVEL AND EFFICIENT SYNTHESIS OF FLUOROMETHYL PHENYL SULFONE AND ITS USE AS A FLUOROMETHYL WITTIG EQUIVALENT[J]. ChemInform, 1985. DOI:10.1002/chin.198536160.
[3] GOUVERNEUR V, LOZANO ó. Preparation of Chiral α-Monofluoromethyl Amines Using Fluoromethyl Phenyl Sulfone[C]. 1900. DOI:10.1055/sos-SD-203-00578.
|
|
|