| Identification | More | [Name]
Trazodone | [CAS]
19794-93-5 | [Synonyms]
8-[3-[4-(3-chlorophenyl)piperazin-1-yl]propyl]-6,8,9-triazabicyclo[4.3.0]nona-2,4,9-trien-7-one
desyrel
TRAZODONE
trazon
trialodine
Trazodone Hydrochloride 2-[3-[4-(3-Chlorophenyl)-1-piperazinyl]propyl]-1,2,4-tri
Trazodone (base and/or unspecified salts) | [EINECS(EC#)]
243-317-1 | [Molecular Formula]
C19H22ClN5O | [MDL Number]
MFCD00242886 | [Molecular Weight]
371.86 | [MOL File]
19794-93-5.mol |
| Hazard Information | Back Directory | [Chemical Properties]
Brown Oil | [Originator]
Trittico,Angelini,Italy,1972 | [Uses]
Antidepressant | [Uses]
It is believed that trazodone, in therapeutic doses, inhibits the neuronal reuptake of sero�tonin. It is not a MAO inhibitor or a CNS stimulator. It has a minor influence on the reup�take of norepinephrine and dopamine. In addition, it does not bind with cholinergic or
α-adrenergic receptors. | [Definition]
ChEBI: An N-arylpiperazine in which one nitrogen is substituted by a 3-chlorophenyl group, while the other is substituted by a 3-(3-oxo[1,2,4]triazolo[4,3-a]pyridin-2(3H)-yl)propyl group. | [Indications]
Trazodone (Apothecon) is also classified as an antidepressant
agent. It is a selective serotonin reuptake inhibitor
(SSRI), partial agonist at postsynaptic 5-HT1A
receptors, and exhibits α-adrenoceptor blocking actions.
Trazodone may cause priapism and enhance libido,
and it prolongs nocturnal erections. This drug has been
used both orally and by intracavernosal injection. It can
be used alone or in combination with yohimbine.
Overall, trazodone has not been as effective in treating
ED as other available agents. However, it may be an option
for selected patients, particularly those with performance
anxiety or low libido. | [Manufacturing Process]
In an initial step, 2-chloropyridine is reacted with semicarbazide to give s_x0002_triazolo-[4,3-a]-pyridine-3-one.
To a boiling solution of 6.7 grams s-triazolo-[4,3-a]-pyridine-3-one in 80 ml
dioxane, there is added 2.4 grams 50% NaH. The mixture is refluxed during 1
hour under stirring, then 13.5 grams 1-(3-chloropropyl)-4-m�chlorophenylpiperazine is added. The mixture is refluxed under stirring for 20
hours, cooled, diluted with an equal volume of ether, the sodium chloride
filtered out, and ethereal HCl added. The solid which precipitates is filtered out and crystallized from 95% alcohol. Yield is 13.5 grams, MP 223°C.
The following is an alternative method of preparation: 1 gram 2-(γ-
chloropropyl)-s-triazolo-[4,3-a]-pyridine-3-one and 5 ml saturated ammonia
alcoholic solution are heated for 5 hours in a closed tube at 100°C. The
contents of the tube are cooled, the ammonium chloride filtered out and the
solvent is removed. There remains a residue of 0.9 grams 2-(γ-aminopropyl)-
s-triazolo-[4,3-a]-pyridine-3-one.
This residue is dissolved in isopropyl alcohol and 1 gram N-bis-chloroethyl�aniline is added to it. The mixture is refluxed for 3 hours. The solvent is
removed at a reduced pressure, the residue is treated with 50% potassium
carbonate, and extracted with ether. By treating with ethereal hydrochloric
acid, 2-N'-m-chlorophenylpiperazino-propyl-s-triazole[4,3-a]pyridine-3-one
hydrochloride is precipitated; MP 223°C. | [Brand name]
Beneficat;Bimaran;Deprax;Devidone;Manegan;Molipaxin;Pragmarel;Pragmazone;Taxagon;Thittico;Thombran;Thromban;Tombran;Tramensan;Tritico;Trittico. | [Therapeutic Function]
Tranquilizer | [World Health Organization (WHO)]
Trazodone, an antidepressant indicated for the treatment of a
wide range of depressive illness, was introduced in 1973. Although it is registered
for use in many countries with highly evolved regulatory authorities, approval for
registration was not granted in Norway because of a suspicion of carcinogenicity
in a two-year rat study. | [Biological Functions]
Trazodone (Desyrel) was introduced in the early 1980s
as a second-generation antidepressant. It blocks the
neuronal reuptake of serotonin and is an antagonist at
the 5HT2-receptor. Also, its major metabolite, mchlorophenylpiperazine
(mCPP), is a postsynaptic serotonin
receptor agonist. When compared to the TCAs,
trazodone is relatively free of antimuscarinic side effects,
but it does block the α-adrenoceptor. Common
side effects include marked sedation, dizziness, orthostatic
hypotension, and nausea. Priapism is
an uncommon but serious side effect requiring surgical
intervention in one-third of the cases reported. Because
of trazodone’s sedating quality, it is often used in low
doses to counter the insomnia associated with the
newer antidepressants, such as the SSRIs. | [Mechanism of action]
Trazodone acts as an antagonist at 5-HT2A receptors and is a weak inhibitor of 5-HT reuptake at the
presynaptic neuronal membrane, potentiating the synaptic effects of 5-HT. Its mechanism of action is
complicated by the presence of its metabolite, m-chlorophenylpiperazine, which is a 5-HT2C
agonist. At therapeutic dosages, trazodone does not appear to influence the reuptake of dopamine or NE
within the CNS. It has little anticholinergic activity and is relatively devoid of toxic cardiovascular effects. The
increase in serotonergic activity with long-term administration of trazodone decreases the number of
postsynaptic serotonergic (i.e., 5-HT2) and β-adrenergic binding sites in the brains of animals, decreasing the
sensitivity of adenylate (or adenylyl) cyclase to stimulation by β-adrenergic agonists. It has been suggested
that postsynaptic serotonergic receptor modification is mainly responsible for the antidepressant action
observed during long-term administration of trazodone. Trazodone does not inhibit MAO and, unlike
amphetamine-like drugs, does not stimulate the CNS.
Trazodone is rapidly and almost completely absorbed from the GItract following oral administration, with an
oral bioavailability of approximately 65%. Peak plasma concentrations of trazodone occur
approximately 1 hour after oral administration when taken on an empty stomach or 2 hours when taken with
food. At steady state, its plasma concentrations exhibit wide interpatient variation.
Trazodone is extensively metabolized in the liver by N-dealkylation to its primary active metabolite,
m-chlorophenylpiperazine (m-CPP), which subsequently undergoes aromatic hydroxylation to
p-hydroxy-m-CPP. In vitro studies indicate that CYP3A4 is the major isoform involved in the
production of m-CPP from trazodone (and CYP2D6 to a lesser extent). The p-hydroxy-m-CPP and
oxotriazolopyridine-propionic acid (the major metabolite excreted in urine) are conjugated with glucuronic
acid. Less than 1% of a dose is excreted unmetabolized. | [Clinical Use]
Trazodone is a phenylpiperazine–triazolopyridine antidepressant that is structurally unrelated to most of the
other antidepressant classes.Trazodone is used primarily in the treatment of insomnia, mental depression, or depression/anxiety disorders.
The drug also has shown some efficacy in the treatment of benzodiazepine or alcohol dependence, diabetic
neuropathy, and panic disorders. | [Synthesis]
Trazodone, 2-[3-[4-(m-chlorophenyl)-1-piperazineyl]propyl]-s-triazolo[4,3-a]
piridine-3(2H)-one (7.3.8), is synthesized from 2-chloropiridine, the reaction of which
with semicarbazide gives s-triazolo-3-one[4,3-a]pyridine (7.3.7). Alkylation of this prod�uct using 1-(3-chloropropyl)-4-(3-chlorophenyl)piperazine gives trazodone (7.3.8)
[61,62].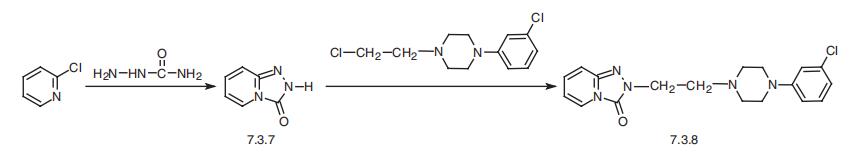
| [Drug interactions]
Trazodone possesses serotonergic activity; therefore, the possibility of developing 5-HT syndrome should be
considered in patients who are receiving trazodone and other SSRIs or serotonergic drugs concurrently.
When trazodone is used concurrently with drugs metabolized by CYP3A4, caution should be used to avoid
excessive sedation. Trazodone can cause hypotension, including orthostatic hypotension and syncope;
concomitant administration of antihypertensive therapy may require a reduction in dosage of the
antihypertensive agent. | [storage]
Store at -20°C |
|
| Company Name: |
Molsyns Research
|
| Tel: |
+91-9586858886 +91-9586858886 |
| Website: |
www.molsyns.com |
| Company Name: |
Niksan Pharmaceutical
|
| Tel: |
+91-9537871777 +91-9537871777 |
| Website: |
www.niksanpharmaceutical.in |
|