| Identification | More | [Name]
1,1-Dimethoxy-N,N-dimethyl-1-butanamine | [CAS]
19718-92-4 | [Synonyms]
(4,4-DIMETHOXY-BUTYL)-DIMETHYL-AMINE
4-(DIMETHYLAMINO)BUTANAL DIMETHYL ACETAL
4-DIMETHYLAMINO BUTYALDEHYDE DIETHYLACETAL
4-DIMETHYLAMINO BUTYALDEHYDE DIMETHYLACETAL
4-(DIMETHYL AMINO)BUTYRALDEHYDE DIMETHYL ACETAL
N,N-Dimethyl-4-aminobutanal dimethyl acetal
1-Butanamine,4,4-dimethoxy-N,N-dimethyl-
N,N-Dimethyl-4,4-Dimethoxy-1-Butanamine
4,4-Diethoxy-N,N-DimethhylButylamine(ForSumatriptan)
4-(N,N-Dimethylamino)butanal dimethyl acetal (intermediate of triptans)
4-(N,N-DIMETHYLAMINO)BUTANAL DIMETHYL ACETAL (FOR TRIPTANS)
4-(N,N-dimethyl amino)butanal dimethyl
4-DIMETHYLAMINE BUTYALDEHYDE DIMETHYL ACETAL, 99% ( FOR SUMATRIPTAN )
4,4-Diethoxy-N,N-dimethhyl butylamine
N,N-dimethylamino-butyraldehyde dimethyl acetal | [EINECS(EC#)]
606-363-7 | [Molecular Formula]
C8H19NO2 | [MDL Number]
MFCD06411126 | [Molecular Weight]
161.24 | [MOL File]
19718-92-4.mol |
| Chemical Properties | Back Directory | [Boiling point ]
164°C | [density ]
0.892 | [refractive index ]
1.4200-1.4240 | [Fp ]
25°C | [storage temp. ]
Inert atmosphere,2-8°C | [solubility ]
Chloroform (Sparingly), Ethyl Acetate (Slightly) | [form ]
clear liquid | [pka]
9.71±0.28(Predicted) | [color ]
Colorless to Light yellow | [Stability:]
Moisture Sensitive | [InChI]
InChI=1S/C8H19NO2/c1-9(2)7-5-6-8(10-3)11-4/h8H,5-7H2,1-4H3 | [InChIKey]
WDZKKBDOGYBYBG-UHFFFAOYSA-N | [SMILES]
C(N(C)C)CCC(OC)OC | [CAS DataBase Reference]
19718-92-4(CAS DataBase Reference) |
| Hazard Information | Back Directory | [Chemical Properties]
Pale Brown Oil.
| [Uses]
4-?(N,?N-?Dimethylamino)?butanal Dimethyl Acetal is a reactant used in the synthesis of hallucinogenic tryptamines. | [General Description]
4-(N,N-Dimethylamino)butanal Dimethyl Acetal is the acetal of 4-(N,N-dimethylamino)butanal. It is soluble in organic solvents and can be used as a building block for various organic syntheses, such as the synthesis of amides or sulfones. 4-(N,N-Dimethylamino)butanal Dimethyl Acetal has a high yield and can be prepared in one step by reacting methanesulfonic acid with dimethyl acetaldehyde. This reaction requires sulfuric acid and methane as catalysts. | [Synthesis]
synthesis of 4-(N,N-Dimethylamino)butanal dimethyl acetal: 4- Chlorobutanal dimethyl acetal (2) (100 g, 0.655 mol) was dissolved in aqueous dimethyl amine solution (200 mL) and the solution is stirred for 15 min at ambient temperature. The reaction mixture was then warmed to 50 oC and stirred for 3 h. After the reaction mixture was cooled to room temperature, the product was extracted with methylene chloride (2 × 250 mL). The combined organic layers were washed with 5 % NaHCO3 solution (2 × 100 mL) and brine solution (2 × 100 mL). The organic layer was evaporated and the residue was distilled to afford 88 g (84 %) of 4-(N,Ndimethylamino)butanal dimethyl acetal as a colourless liquid with 99.6 % purity by GC: b.p. 40 oC/1 mm Hg.
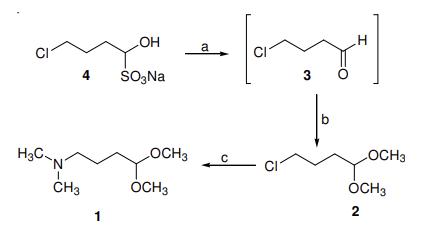
(a) (i) Sodium carbonate, dichloromethane, 5 oC, 0.5 h (ii) Methanol, conc. H2SO4, room temperature, 3 h, 87 %. (b) Aqueous dimethyl amine solution (30 %), 50 oC, 3 h, 76.0 %
1H NMR (CDCl3, 200 MHz): δ 1.47-1.63 (m, 4H), 2.21 (s, 6H), 2.24 (t, J = 7.0 Hz, 2H), 3.31 (s, 6H), 4.37 (t, J = 5.4 Hz, 2H). IR (cm-1): 2945 (-CH2-), 2816 (-CH-), 1464 (C-N), 1074 (-C-O-). Mass: m/z 162.5 (M+1). |
|
|