| Identification | More | [Name]
tert-Butyl 4-aminobenzoate | [CAS]
18144-47-3 | [Synonyms]
4-AMINOBENZOIC ACID TERT-BUTYL ESTER
BUTTPARK 14\02-67
TERT-BUTYL 4-AMINOBENZOATE | [EINECS(EC#)]
629-537-4 | [Molecular Formula]
C11H15NO2 | [MDL Number]
MFCD00665790 | [Molecular Weight]
193.24 | [MOL File]
18144-47-3.mol |
| Chemical Properties | Back Directory | [Melting point ]
108-110 °C | [Boiling point ]
322.4±15.0 °C(Predicted) | [density ]
1.078±0.06 g/cm3(Predicted) | [storage temp. ]
Keep in dark place,Inert atmosphere,Room temperature | [form ]
powder to crystal | [pka]
2.43±0.10(Predicted) | [color ]
White to Light yellow to Light orange | [BRN ]
2803178 | [CAS DataBase Reference]
18144-47-3(CAS DataBase Reference) |
| Safety Data | Back Directory | [Hazard Codes ]
Xi | [Risk Statements ]
R36/37/38:Irritating to eyes, respiratory system and skin . | [Safety Statements ]
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice .
S36:Wear suitable protective clothing . | [WGK Germany ]
3
| [F ]
10-34 | [Hazard Note ]
Irritant | [HS Code ]
29224999 |
| Hazard Information | Back Directory | [Uses]
tert-Butyl 4-aminobenzoate(18144-47-3) can be used to synthesise structural units for antifolate and a novel liver-targeted coupling of PSN-357, a glycogen phosphorylase inhibitor for the treatment of diabetes. It can also be used in the synthesis of tert-butyl 4-aminobenzoate n-substituted derivatives[1].
| [reaction suitability]
reaction type: solution phase peptide synthesis | [Synthesis]
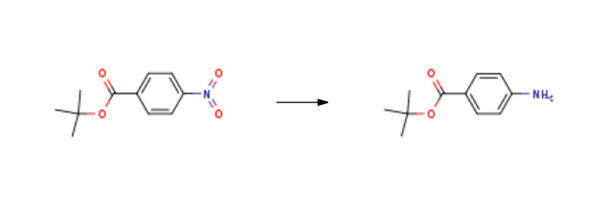
tert-Butyl 4-aminobenzoate(18144-47-3) is synthesised using tert-butyl 4-nitrobenzoate as raw material by chemical reaction. The specific synthesis steps are as follows:
Tert-butyl 4-nitrobenzoate (4.75 g, 21.279 mmol) was dissolved in MeOH (200 ml) and added with CAUTION was Pd/C (475 mg, 0.1 equiv. w/w) and the flask evacuated prior to purging with N2, re-evacuation and purging with H2. The mixture was stirred under H2 for 5 hours until complete. The mixture was filtered through celite and evaporated to a white solid (4.11 g, 21.269 mmol, quant.). Rf 0.29 [20% EtOAc in Hexane]. Mp. I l l - 113 0C. 1H NMR (400 MHz, CDCl3) δ 7.79 (2H, dt, J=8.6, 2.3 Hz, 2ArH), 6.61 (2H, dt, J=8.6, 2.3 Hz, 2ArH), 4.00 (2H, br s, NH2), 1.56 (9H, s, C(CH3)3) ppm. 13C NMR (100 MHz, CDCl3) δ 165.9 (C=O), 150.4 (ArC), 131.3 (ArCH), 121.7 (ArC), 113.7 (ArCH), 80.0 (C(CH3)3), 28.3 (C(CH3)3) ppm. IR (neat) υmax 3415, 3345, 3235, 2972, 1682, 1598, 1367, 1287, 1154, 1115 cm 1. HRMS mJz calc. C11H15NO2 [M+l] 194.1176, found [M+l] 194.1179.
| [References]
[1] V. BAVETSIAS E. H. Synthesis of N-substituted derivatives of tert-butyl 4-aminobenzoate via a palladium-catalysed reaction[J]. Journal of Chemical Research-s, 2000. DOI:10.3184/030823400103168100. |
|
|