| Identification | More | [Name]
PYRIDINE-3-SULFONYL CHLORIDE HYDROCHLORIDE | [CAS]
16133-25-8 | [Synonyms]
3-CHLOROSULFONYL-PYRIDINIUM, CHLORIDE
3-PYRIDINESULFONYL CHLORIDE, HCL
3-PYRIDINESULFONYL CHLORIDE HYDROCHLORIDE
PYRIDINE-3-SULFONYL CHLORIDE HYDROCHLORIDE
PYRIDINE-3-SULPHONYL CHLORIDE HYDROCHLORIDE
3-Pyridine sulphonyl chloride | [EINECS(EC#)]
688-270-1 | [Molecular Formula]
C5H5Cl2NO2S | [MDL Number]
MFCD04035552 | [Molecular Weight]
214.07 | [MOL File]
16133-25-8.mol |
| Chemical Properties | Back Directory | [Melting point ]
144 °C | [Boiling point ]
284℃ | [density ]
1.488 | [Fp ]
126℃ | [storage temp. ]
Inert atmosphere,2-8°C | [solubility ]
Chloroform (Sparingly), Methanol (Slightly) | [form ]
liquid | [pka]
-1.77±0.11(Predicted) | [color ]
Colourless to light yellow | [Stability:]
Moisture Sensitive | [InChI]
InChI=1S/C5H4ClNO2S/c6-10(8,9)5-2-1-3-7-4-5/h1-4H | [InChIKey]
CDRNYKLYADJTMN-UHFFFAOYSA-N | [SMILES]
C1=NC=CC=C1S(Cl)(=O)=O | [LogP]
1.75 | [CAS DataBase Reference]
16133-25-8(CAS DataBase Reference) |
| Safety Data | Back Directory | [Hazard Codes ]
Xi | [Risk Statements ]
R34:Causes burns. | [Safety Statements ]
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice .
S36/37/39:Wear suitable protective clothing, gloves and eye/face protection . | [RIDADR ]
3261 | [WGK Germany ]
3 | [HazardClass ]
IRRITANT | [PackingGroup ]
Ⅲ | [HS Code ]
2933399990 |
| Hazard Information | Back Directory | [Uses]
3-pyridinesulfonyl Chloride is a reagent used in the synthesis of pyrimidine derivatives with anti-proliferative activity against negative breast cancer cells. | [Application]
Pyridine-3-sulfonyl chloride is an essential medical intermediate and is widely applied to the synthesis of medicines, wherein the pyridine-3-sulfonyl chloride is mainly used for preparing TAK-438 (vonoprazan fumarate), which is a novel orally active potassium-competitive acid blocker, developed as an antisecretory drug.
| [Synthesis]
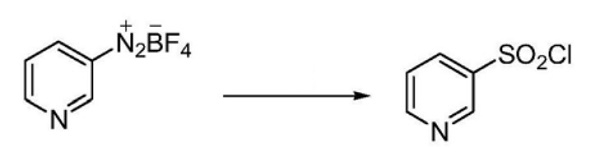
Add thionyl chloride to the water, cool to about 0-5°C, and add cuprous chloride. Fluoboric acid diazonium salt was added to the solution and reacted at 0-5°C overnight. The reaction solution was extracted with dichloromethane, combining the organic layers. The organic layer was washed once with saturated aqueous sodium bicarbonate solution, once with water, and finally once with saturated brine. The product was dried over anhydrous sodium sulfate and filtered, and the filtrate was concentrated to remove methylene chloride to obtain the product pyridine-3-sulfonyl chloride with a yield of 90.7%.
|
|
|