| Identification | Back Directory | [Name]
4-BROMOMETHYL-PIPERIDINE-1-CARBOXYLIC ACID TERT-BUTYL ESTER | [CAS]
158407-04-6 | [Synonyms]
1-Boc-4-BromomethyL
1-Boc-4-broMoMethylpiperi...
1-BOC-4-BROMOMETHYLPIPERIDINE
N-BOC-4-BROMOMETHYL-PIPERIDINE
1-N-BOC-4-BROMOMETHYLPIPERIDINE
1-Boc-4-(bromoMmethyl)piperidine
4-Bromomethyl-1-(tert-butoxycarbonyl)piperidine
tert-Butyl 4-(bromomethyl)piperidine-1-carboxylate
4-BROMOMETHYL-PIPERIDINE-1-CARBOXYLIC ACID TERT-BUTYL ESTER
4-Bromomethyl-piperidine-1-carboxylic acid tert-butyk ester
4-Bromomethylpiperidine-1-carboxylic acid tert-butyl ester 95%
1-Piperidinecarboxylic acid, 4-(bromomethyl)-, 1,1-dimethylethyl ester | [Molecular Formula]
C11H20BrNO2 | [MDL Number]
MFCD04115538 | [MOL File]
158407-04-6.mol | [Molecular Weight]
278.19 |
| Chemical Properties | Back Directory | [Boiling point ]
318.3±15.0 °C(Predicted) | [density ]
1.270±0.06 g/cm3(Predicted) | [storage temp. ]
Room temperature. | [pka]
-1.91±0.40(Predicted) | [InChIKey]
YGJXBTRLYHCWGD-UHFFFAOYSA-N |
| Hazard Information | Back Directory | [Uses]
4-Bromomethypiperidine-1-carboxylic acid tert-butyl ester can be used as organic synthesis intermediate and pharmaceutical intermediate, mainly in laboratory research and development process and chemical production process. | [Synthesis]
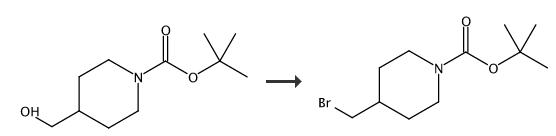
4-Bromomethypiperidine-1-carboxylic acid tert-butyl ester. 4-N-Boc-piperidine-methanol (200 mg, 0.93 mmol) was dissolved in diethyl ether (9 mL) and carbon tetrabromide (370 mg, 1.1 mmol) and PPh3 (292 mg, 1.1 mmol) were added at rt. The reaction was allowed to stir for 18 h at rt and filtered over a pad of celite. The filtrate was concentrated and purified by flash chromatography (hexane/EtOAc, 1:0 ?ú 4:1) to give the title compound. Yield 55 mg. 1 (400 MHz, DMSO-d6) |? ppm 4.02-3.98 (m, 2 H), 3.47 (d, 2 H), 2.78-2.65 (m, 2 H), 1.89-1.74 (m, 3 H), 1.45 (s, 9 H), 1.12-0.98 (m, 2 H). |
|
|