| Identification | More | [Name]
9,10-Diphenylanthracene | [CAS]
1499-10-1 | [Synonyms]
9,10-DIPHENYLANTHRACENE
9,10-diphenyl-anthracen
anthracene,9,10-diphenyl-
9,10-DIPHENYLANTHRACEN 97%
9,10-DIPHENYLENEANTHRACENE OEKANAL, 250
Bis(2,4,6-Trichlorophenyl)EthanedioateTcpo
9,10-Diphenylanthracene(blue) (DPA)
9,10-Diphenylanthracene, scintillation grade, 99+% | [EINECS(EC#)]
216-105-1 | [Molecular Formula]
C26H18 | [MDL Number]
MFCD00001253 | [Molecular Weight]
330.42 | [MOL File]
1499-10-1.mol |
| Chemical Properties | Back Directory | [Appearance]
slightly yellow powder | [Melting point ]
245-248 °C(lit.)
| [Boiling point ]
485°C(lit.) | [density ]
1.1398 (estimate) | [refractive index ]
1.8430 (estimate) | [storage temp. ]
Store below +30°C. | [form ]
Fine Crystalline Powder | [color ]
Light yellow to beige | [Stability:]
Stable. Combustible. Incompatible with strong oxidizing agents. | [Water Solubility ]
INSOLUBLE | [λmax]
393nm(Cyclohexane)(lit.) | [BRN ]
1914010 | [InChIKey]
FCNCGHJSNVOIKE-UHFFFAOYSA-N | [CAS DataBase Reference]
1499-10-1(CAS DataBase Reference) | [NIST Chemistry Reference]
Anthracene, 9,10-diphenyl-(1499-10-1) | [EPA Substance Registry System]
1499-10-1(EPA Substance) |
| Safety Data | Back Directory | [Hazard Codes ]
Xi | [Risk Statements ]
R36/37/38:Irritating to eyes, respiratory system and skin . | [Safety Statements ]
S22:Do not breathe dust .
S24/25:Avoid contact with skin and eyes .
S36/37:Wear suitable protective clothing and gloves .
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice . | [RIDADR ]
2811 | [WGK Germany ]
3
| [RTECS ]
SS6825000 | [TSCA ]
Yes | [HS Code ]
29029080 |
| Hazard Information | Back Directory | [Chemical Properties]
slightly yellow powder | [Uses]
9,10-Diphenylanthracene acts as a sensitizer in chemiluminescence. It is used in light sticks in order to produce blue light. It is also useful in blue organic light-emitting diodes (OLEDs) and OLED-based displays. Further, it plays an important role in the preparation of 9,10-difluoro-9,10-diphenyl-9,10-dihydro-anthracene. In addition to this, it is used as an organic semiconductor. | [Application]
9,10-Diphenylanthracene is a polycyclic aromatic hydrocarbon and a useful organic semiconductor. 9,10-Diphenylanthracene is also a sensitiser in chemiluminescence. It has applications related to OLEDs and OLED-based displays. | [Definition]
ChEBI: A member of the class of anthracenes that is anthracene in which both of the hydrogens on the central ring are substituted by phenyl groups. | [General Description]
9,10-Diphenylanthracene, an aromatic hydrocarbon, is a blue light emitting material that is used for the measurement of fluorescence quantum yields in dilute solutions. Its derivatives show potential candidature in organic light emitting diode (OLED) devices. It is also used as a fluorophore for the study of peroxyoxalate chemiluminescence (POCL). | [Synthesis]
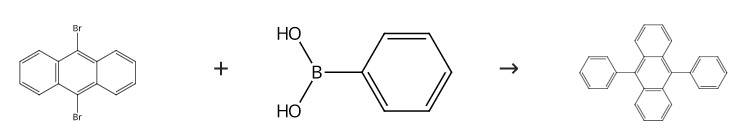
9, 10 – Diphenylanthracene was synthesized via a multistep synthesis using 9, 10 – dibromoanthracene, a Suzuki coupling was performed using phenylboronic acid over a tetrakis(triphenylphosphine)palladium(0) catalyst to form the title compound:
The mixture of 1,3-dibromobenzene (236 mg, 1.0 mmol), phenylboronic acid (271 mg, 2.2 mmol), and Na2CO3 (424 mg, 4 mmol) was taken up in DMF (2 mL) and then ARF-Pd (300 mg, 0.01 mmol Pd) was added. The mixture was heated under N2 at 110 °C for 2.5 h. After cooling, the mixture was diluted with cold H2O (10 mL) and filtered through a cotton bed. The filtrate was extracted with Et2O (3 × 20 mL) and the combined organic extracts were washed with brine (10 mL), dried (Na2SO4), and evaporated. The residue was purified by column chromatography (silica gel, PE) to give 9,10-Diphenylanthracene (4d), yield 90%. Mp 245-247 °C (Lit.36 245-48 °C). IR (Nujol): 1458, 1377 cm-1. 1H NMR (300 MHz, CDCl3): δ = 7.72-7.66 (m, 4 H), 7.62-7.46 (m, 10 H), 7.34-7.29 (m, 4 H). 13C NMR (75 MHz, CDCl3): δ = 139.1, 137.1, 131.3, 129.8, 128.4, 127.4, 126.9, 125.0.
 | [Purification Methods]
Crystallise the anthracene from acetic acid or xylene [Baumstark et al. J Org Chem 52 3308 1987]. [Beilstein 5 IV 2807.] |
|
|