| Identification | More | [Name]
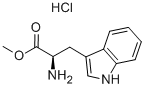
D-Tryptophan methyl ester hydrochloride | [CAS]
14907-27-8 | [Synonyms]
D-TRYPTOPHAN METHYL ESTER
D-TRYPTOPHAN METHYL ESTER HCL
D-TRYPTOPHAN METHYL ESTER HYDROCHLORIDE
D-TRYPTOPHAN METHYL ESTER HYDROCHLORIDE SALT
D-TRYPTOPHAN-OME HCL
H-D-TRP-OME HCL
D-TRYPTOPHAN METHYL ESTER HYDROCHLORIDE 99%
D-TRP.OME.HCL
(S)-2-Amino-3-(1H-indol-3-yl)-propionic acid methyl ester hydrochloride
Methyl (2R)-2-amino-3-(1H-indol-3-yl)propanoate hydrochloride
D-Tryptophane methyl ester hydrochloride
Trp-OMe
(R)-Tryptophan Methyl Ester Monohydrochloride
Methyl D-Tryptophanate Hydrochloride | [EINECS(EC#)]
639-469-7 | [Molecular Formula]
C12H15ClN2O2 | [MDL Number]
MFCD00038992 | [Molecular Weight]
254.71 | [MOL File]
14907-27-8.mol |
| Chemical Properties | Back Directory | [Appearance]
Off-White Crystalline Solid | [Melting point ]
213-216 °C(lit.)
| [density ]
1.337g/cm3 at 20℃ | [refractive index ]
-19 ° (C=5, MeOH) | [storage temp. ]
2-8°C | [solubility ]
DMSO, (Slightly), Methanol (Slightly), Water (Slightly) | [form ]
Solid | [color ]
White to Off-White | [optical activity]
[α]20/D 18°, c = 5 in methanol | [Water Solubility ]
Soluble in water (150 mg/ml), dimethyl sulfoxide and methanol (50 mg/ml). | [InChI]
InChI=1/C12H14N2O2.ClH/c1-16-12(15)10(13)6-8-7-14-11-5-3-2-4-9(8)11;/h2-5,7,10,14H,6,13H2,1H3;1H/t10-;/s3 | [InChIKey]
XNFNGGQRDXFYMM-MEQOOBBNNA-N | [SMILES]
C12C=CC=CC=1NC=C2C[C@@H](N)C(=O)OC.Cl |&1:10,r| | [LogP]
1.089 | [CAS DataBase Reference]
14907-27-8(CAS DataBase Reference) |
| Hazard Information | Back Directory | [Description]
D-Tryptophan Methyl Ester Hydrochloride is an intermediate in the synthesis of Tryptophan derivatives. Soluble in water (150 mg/ml), dimethyl sulfoxide and methanol (50 mg/ml). Incompatible with strong oxidizing agents. Store under room temperature. | [Chemical Properties]
Off-White Crystalline Solid | [Uses]
Intermediate for the synthesis of Tryptophan derivatives. | [Application]
The Pictet–Spengler reaction of D-Tryptophan methyl ester hydrochloride with piperonal in various solvents has been extensively studied, the solvent-dependence of stereoselectivities could be principally attributed to the solubility difference between cis and trans products 5-HCl in the used solvent, the best stereoselectivity (cis/trans = 99:1) was obtained using nitromethane or acetonitrile as the solvent. Cialis, 12a-epi-Cialis, deuterium-labeled, 3,3,12a-d3-Cialis, and 3,3,12a-d3-12a-epi-Cialis could be efficiently synthesized from D-Tryptophan methyl ester hydrochloride[1].
| [reaction suitability]
reaction type: solution phase peptide synthesis | [Synthesis]
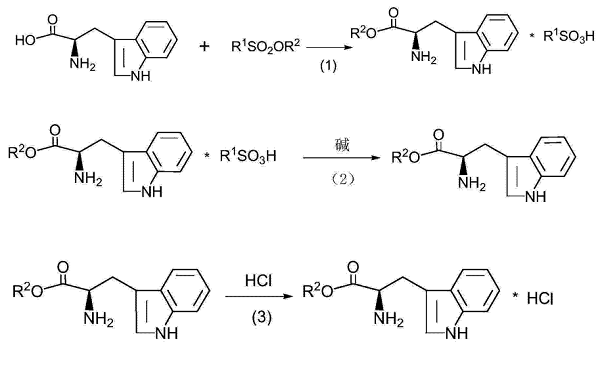
D-tryptophan and methyl sulfonate are reacted in a solvent at 25 to 100°C to generate D-tryptophan methyl ester sulfonate. Dissolve the obtained D-tryptophan methyl ester sulfonate in water and adjust the pH to alkaline with alkali to convert it into D-tryptophan methyl ester. After the reaction, extract with an organic solvent, add a desiccant for dehydration, and obtain an extract containing D-tryptophan methyl ester. Finally, dry hydrogen chloride gas is passed into the obtained extract containing D-tryptophan methyl ester for reaction to obtain D-tryptophan methyl ester hydrochloride.
| [References]
[1] Xiao-Xin Shi. “Highly stereoselective Pictet–Spengler reaction of d-tryptophan methyl ester with piperonal: convenient syntheses of Cialis (Tadalafil), 12a-epi-Cialis, and their deuterated analogues.” Tetrahedron, asymmetry 19 4 (2008): Pages 435-442.
|
|
|