| Identification | More | [Name]
Glatiramer acetate | [CAS]
147245-92-9 | [Synonyms]
GLATIRAMER ACETATE
l-glutamic acid polymer with l-alanine, l-lysine and l-tyrosine, acetate (salt) | [EINECS(EC#)]
814-981-5 | [Molecular Formula]
C25H45N5O13 | [MDL Number]
MFCD04113403 | [MOL File]
147245-92-9.mol | [Molecular Weight]
623.65 |
| Hazard Information | Back Directory | [Uses]
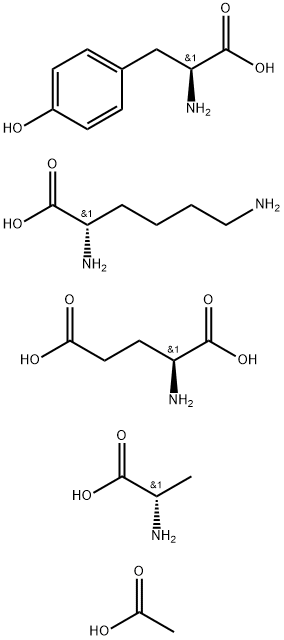
Glatiramer acetate is a random basic synthetic copolymer of L-alanine, L-lysine, l-glutamic acid and L-tyrosine in a molar ratio of 6:1.9:4.7:1. Glatiramer acetate is an immunomodulator used in treatment of multiple sclerosis. | [Originator]
Copaxone,Teva Pharmaceuticals,Israel | [Manufacturing Process]
Glatiramer Acetate is water soluble copolypeptide with molecular weight
15,000-25,000.
Copolymer is prepared by copolymerization of the N-carboxyanhydrides of
tyrosine, alanine, lysine, and glutamic acid. The polymerisation was carried
out at ambient temperature in anhydrous dioxane with diethylamine as
initiator. Glatiramer Acetate have the ratio alanin:glutamic acid:lysine:tyrosine
= 1:6:4.54:2. | [Brand name]
Copaxone (Teva). | [Therapeutic Function]
Immunomodulator | [Biological Activity]
Glatiramer acetatea myelin basic protein mimeticis an immunomodulator used for treatment of Multiple Sclerosis. Glatiramer acetate is a mixture of synthetic peptides randomly composed of glutamic acidlysinealanineand tyrosine. It reduces relapse rate and disease progression in Multiple Sclerosis. Its mechanism of action is not fully understood. Glatiramer acetate generates anti-inflammatory Th2 cellswhich produce neurotrophic factors. | [Clinical Use]
Immunomodulating drug:
Treatment for patients at a high risk of developing
multiple sclerosis and for reduction in relapses in
ambulatory patients | [Drug interactions]
Potentially hazardous interactions with other drugs
None known | [Metabolism]
A substantial fraction of a subcutaneous dose of
glatiramer is believed to be hydrolysed locally. Some of
the injected dose is also presumed to enter the lymphatic
system, either intact or partially hydrolysed. |
|
|