| Identification | More | [Name]
1,3-BIS(2,4,6-TRIMETHYLPHENYL)IMIDAZOLIUM CHLORIDE | [CAS]
141556-45-8 | [Synonyms]
1,3-(2,4,6-TRIMETHYLPHENYL)IMIDAZOLIUM CHLORIDE
1,3-BIS(2,4,6-TRIMETHYLPHENYL)-4,5-DIHYDROIMIDAZOLIUM CHLORIDE
1,3-BIS-(2,4,6-TRIMETHYLPHENYL)IMIDAZOLINIUM CHLORIDE
1,3-BIS(2,4,6-TRIMETHYLPHENYL)IMIDAZOLIUM CHLORIDE
1,3-DIMESITYLIMIDAZOLIUM CHLORIDE
N,N'-(2,4,6-TRIMETHYLPHENYL)DIHYDROIMADAZOLIUM CHLORIDE
1,3-BIS(2,4,6-TRIMETHYLPHENYL)IMIDAZOLI&
1,3-Bis(2,4,6-trimethylphenyl)imidazoliumchloride,min.95%
1,3-BIS-(2,4,6-TRIMETHYLPHENYL)-1H-IMIDAZOLIUM CHLORIDE
1,3-Bis-(2,4,6-trimethyl-phenyl)-3H-imidazol-1-ium chloride
1,3-Bis(2,4,6-trimethylphenyl)imidazolium chloride, min. 97%
IMes.HCl | [Molecular Formula]
C21H25ClN2 | [MDL Number]
MFCD02684541 | [Molecular Weight]
340.89 | [MOL File]
141556-45-8.mol |
| Chemical Properties | Back Directory | [Appearance]
off-white to beige powder | [Melting point ]
>300 °C (lit.) | [Boiling point ]
499.2°C (rough estimate) | [density ]
1.0279 (rough estimate) | [refractive index ]
1.5940 (estimate) | [storage temp. ]
Keep in dark place,Inert atmosphere,2-8°C | [form ]
Powder | [color ]
Off-white to beige | [Water Solubility ]
Slightly soluble in water. | [InChIKey]
OTOSIXGMLYKKOW-UHFFFAOYSA-M | [CAS DataBase Reference]
141556-45-8(CAS DataBase Reference) |
| Safety Data | Back Directory | [Hazard Codes ]
Xi | [Risk Statements ]
R36/37/38:Irritating to eyes, respiratory system and skin . | [Safety Statements ]
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice .
S36:Wear suitable protective clothing . | [WGK Germany ]
3
| [TSCA ]
No | [HS Code ]
29332900 |
| Hazard Information | Back Directory | [Chemical Properties]
off-white to beige powder | [Uses]
Used as a phosphine-free ligand in various metal-catalyzed coupling reactions, often with advantageous results in difficult cases. For use in the Pd-catalyzed cross-coupling of aryl Grignards with aryl chlorides (Kumada reaction). Many examples have been recorded of the use of NHC ligands in the Suzuki coupling reaction, for examples utilizing 1,3-dimesitylimidazol-2-ylidene, in the coupling of arylboronic acids with relatively unreactive aryl chlorides. | [Preparation]
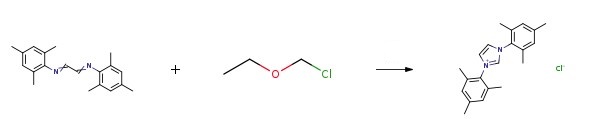
In a flask, the imine(3 g, 10 mmol) was dissolved in tetrahydrofuran(25 ml), followed by dropwise addition of chloromethyl ethyl ether(1.04 g, 11 mmol). the mixture was stirred under N2 at 40 °C for 18 h, and then ethyl ether(25 ml) was added to separate white solid. The solid was filtered and washed with ethyl ether. Finally, the white solid was dried under vacuum affording 1,3-Bis(2,4,6-trimethylphenyl)imidazolium chloride.
| [Reactions]
Precursor to the nucleophilic carbene that serves as a bulky, electron-rich "phosphine mimic" for metal-catalyzed reactions.
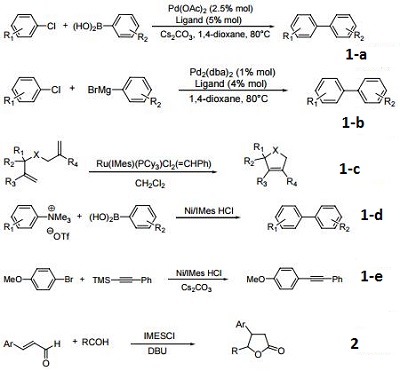
(a) Palladium-catalyzed Suzuki cross-coupling of aryl chlorides.
(b) Palladium-catalyzed Kumada cross-coupling of aryl chlorides.
(c) Ruthenium-carbene catalysts for ring-closing metathesis.
(d) Suzuki coupling of aryltrimethylammonium salts.
(e) Sonogashira coupling of aryl bromides.
Precursor to a nucleophilic carbene that serves as catalyst.
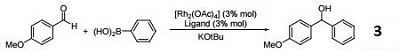
Ligand for arylation of aldehydes.
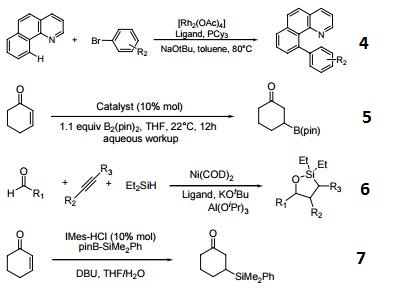
Ligand for carbene catalyzed intermolecular arylation of C-H bonds.
Catalyst for boron conjugate additions to cyclic and acyclic α,β-unsaturated carbonyls.
Ligand for dehydrogenative cyclocondensation of aldehydes, alkynes, and dialkylsilanes.
Precursor for carbene for conjugate silylation of alpha, beta-unsaturated carbonyls.
 |
|
|