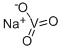| Identification | More | [Name]
Sodium metavanadate | [CAS]
13718-26-8 | [Synonyms]
SODIUM METAVANADATE
SODIUM METAVANADATE(V)
SODIUM MONOVANADATE
SODIUM M-VANADATE
SODIUM VANADATE
SODIUM VANADATE (META)
SODIUM VANADATE(V)
SODIUM VANADIUM OXIDE
metawanadansodowy
monosodiumtrioxovanadate(1-)
sodiumtrioxovanadate(1-)
Sodiumvanadate(NaVO3)
sodiumvanadate(v)(navo3)
vanadate(vo3(1-)),sodium
vanadate(vo31-),sodium
Vanadate,sodium
Vanadicacid(HVO3),sodiumsalt
vanadicacid,monosodiumsalt
Vanadicacidsodiumsalt
SODIUM META VANADATE, 99.9% | [EINECS(EC#)]
237-272-7 | [Molecular Formula]
NaO3V | [MDL Number]
MFCD00011125 | [Molecular Weight]
121.93 | [MOL File]
13718-26-8.mol |
| Chemical Properties | Back Directory | [Appearance]
white to off-white powder | [Melting point ]
600 °C
| [density ]
3.241 g/cm3 | [storage temp. ]
Sealed in dry,Room Temperature | [form ]
Powder | [color ]
White to faintly yellow-beige or pale green | [Odor]
Odorless | [Stability:]
Stable. | [Water Solubility ]
Soluble in water. | [Merck ]
14,8700 | [Exposure limits]
NIOSH: Ceiling 0.05 mg/m3 | [InChI]
InChI=1S/Na.3O.V/q+1;;;-1; | [InChIKey]
CMZUMMUJMWNLFH-UHFFFAOYSA-N | [SMILES]
[V]([O-])(=O)=O.[Na+] | [Uses]
Vanadium is a metallic element that occurs in six oxidation states and numerous inorganic compounds. Some of the more important compounds are vanadium pentoxide (V2O5), sodium metavanadate (NaVO3), sodium orthovanadate (Na3VO4), vanadyl sulfate (VOSO4), and ammonium vanadate (NH4VO3). Vanadium is used primarily as an alloying agent in steels and non-ferrous metals (ATSDR, 1990). Vanadium compounds are also used as catalysts and in chemical, ceramic or specialty applications. | [CAS DataBase Reference]
13718-26-8(CAS DataBase Reference) | [EPA Substance Registry System]
Sodium vanadate (13718-26-8) |
| Safety Data | Back Directory | [Hazard Codes ]
T | [Risk Statements ]
R25:Toxic if swallowed.
R36/37/38:Irritating to eyes, respiratory system and skin . | [Safety Statements ]
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice .
S36/37/39:Wear suitable protective clothing, gloves and eye/face protection .
S45:In case of accident or if you feel unwell, seek medical advice immediately (show label where possible) . | [RIDADR ]
UN 3285 6.1/PG 3
| [WGK Germany ]
3
| [RTECS ]
YW1050000
| [TSCA ]
Yes | [HazardClass ]
6.1 | [PackingGroup ]
II | [HS Code ]
28419000 | [Safety Profile]
Poison by ingestion, intraperitoneal, subcutaneous, and intravenous routes. Experimental reproductive effects. Mutation data reported. When heated to decomposition it emits toxic fumes of Na2O and VOx. See also VANADIUM COMPOUNDS. |
| Hazard Information | Back Directory | [General Description]
Colorless to yellow crystals or cream colored solid. Melting point 630°C. | [Reactivity Profile]
SODIUM VANADATE(13718-26-8) is a moderately strong oxidizing agent [Cotton and Wilkinson]. | [Air & Water Reactions]
Soluble in water. | [Hazard]
Toxic by ingestion. | [Fire Hazard]
Flash point data of this compound are not available. SODIUM VANADATE is probably nonflammable. | [Chemical Properties]
Sodium vanadate, sodium orthovanadate, Na3VO4, white solid, soluble, formed by fusion of vanadium pentoxide and sodium carbonate. Used (1) in inks, (2) in photography, (3) in dyeing of furs, (4) in inoculation of plant life. | [Chemical Properties]
white to off-white powder | [Definition]
ChEBI: An inorganic sodium salt having metavanadate as the counterion. | [Preparation]
sodium metavanadate synthesis: Dissolve vanadium pentoxide in sodium hydroxide solution, crystallized by concentration, that is, the finished product of sodium metavanadate.
V2O5+2NaOH→2NaVO3+H2O | [Health Hazard]
Vanadium pentoxide and sodium metavanadate have a toxicity rating of 5, equivalent to a probable lethal oral dose in humans of 5-50 mg/kg (Gosselin et al., 1984). The elemental metallic form is considered to be non-toxic.
Stokinger et al. (1953) reported that a 10% solution of sodium metavanadate is a primary irritant to human skin. Saturated solutions of ammonium metavanadate (0.5%) and vanadium pentoxide (0.8% solution) did not irritate the skin. Sjoberg (1951) reported that several workers occupationally exposed to vanadium developed what appeared to be a contact dermatitis and that in one case, skin patch tests produced eczematous lesions indicative of an allergic reaction.
COMMENTS: The NOAEL is derived from a study in which rats were given 0, 5, 10 and 50 ppm sodium metavanadate, in drinking water for 3 months. Impaired kidney function was seen at 50 ppm, and 10 ppm was considered a NOAEL. The Uncertainty Factor of 100 is the product of a 10-fold uncertainty in extrapolating from laboratory animals to humans and a 10-fold uncertainty to protect sensitive individuals. | [Flammability and Explosibility]
Nonflammable |
|
|