| Identification | Back Directory | [Name]
Duloxetine hydrochloride | [CAS]
136434-34-9 | [Synonyms]
LY-248686
Duloxetime
LY-248686 HCl
DULOXETINE HCL
S-Duloxetine HCl
(RS)Duloxetine HCl
LY-248686 hydrochloride
DULOXETINE HYDROCHLORIDE
(RS)-DULOXEINEHYDROCHLORIDE
(S)-DULOXETINE HYDROCHLORIDE
Duloxetine hydrochloride ,99%
Duloxetine HCl and intermediates
Duloxetine for system suitability
Duloxetine hydrochloride solution
DULOXETINE: DULOXETINE HYDROCHLORIDE
(S)-Duloxetine hydrochloride(CyMbalta)
N-Methyl-gaMa-(1-naphthalenyloxy)-2-thiophenepropa
Methanol( Test Duloxetine HCl, 1.0 mg/mL as free base)
N-Methyl-gama-(1-naphthalenyloxy)-2-thiophenepropanamine
(+)-(S)-N-Methyl--(1-naphthyloxy)-2-thiophenepropylamine
(S)-N-Methyl--(1-naphthalenyloxy)-2-thiophenepropanamine
(+)-(S)-N-Methyl-g-(1-naphthyloxy)-2-thiophenepropylamine
(gS)-N-Methyl-g-(1-naphthalenyloxy)-2-thiophenepropanamine
(+)-(S)-N-Methyl--(1-naphthyloxy-d7)-2-thiophenepropylamine
(S)-N-Methyl--(1-naphthalenyloxy-d7)-2-thiophenepropanamine
(+)-(S)-N-Methyl-g-(1-naphthyloxy-d7)-2-thiophenepropylamine
(gS)-N-Methyl-g-(1-naphthalenyloxy-d7)-2-thiophenepropanamine
(+)-(S)-N-Methyl-γ-(1-naphthyloxy)-2-thiophenepropylaMine Hydrochloride
(γS)-N-Methyl-γ-(1-naphthalenyloxy)-2-thiophenepropanaMine Hydrochloride
N-methyl-gamma-(1-naphthalenyloxy)-2-thiophenepropanamine, hydrochloride
(+)-N-Methyl-3-(1-naphthalenyloxy)-3-(2-thienyl)propanamine hydrochloride
(S)-N-Methyl-3-(1-naphthalenyloxy)-3-(2-thienyl) propanamine hydrochloride
N-Methyl-3-(naphthalen-1-yloxy)-3-(thiophen-2-yl)propan-1-aMine hydrochloride
(γS)-N-methyl-γ-(1-naphthalenyloxy)-2-thiophenepropanamine, hydrochloride (1:1)
(S)-N-Methyl-3-(naphthalen-1-yloxy)-3-(thiophen-2-yl)propan-1-aMine hydrochloride
(S)-N,N-DiMethyl-3-(naphthalen-1-yloxy)-3-(thiophen-2-yl)propan-1-aMine hydrochloride
(+)-(S)-n-methyl-g-(1-naphthyloxy)-2-thiophenepropylamine, hydrochloride/duloxetinehcl | [EINECS(EC#)]
200-659-6 | [Molecular Formula]
C18H20ClNOS | [MDL Number]
MFCD06407958 | [MOL File]
136434-34-9.mol | [Molecular Weight]
333.88 |
| Chemical Properties | Back Directory | [Appearance]
White Crystalline Solid | [Melting point ]
118-122°C | [Fp ]
9℃ | [storage temp. ]
-20°C Freezer | [solubility ]
H2O: soluble5mg/mL (clear solution, warmed) | [form ]
powder | [pka]
pKa in DMF-water (66:34): 9.6(at 25℃) | [color ]
white to beige | [optical activity]
[α]/D +116 to +125°, c = 1 in methanol | [Merck ]
14,3465 | [InChI]
InChI=1/C18H19NOS.ClH/c1-19-12-11-17(18-10-5-13-21-18)20-16-9-4-7-14-6-2-3-8-15(14)16;/h2-10,13,17,19H,11-12H2,1H3;1H/t17-;/s3 | [InChIKey]
BFFSMCNJSOPUAY-VOPAOICTNA-N | [SMILES]
C1(=CC=CS1)[C@H](CCNC)OC1=CC=CC2=CC=CC=C12.Cl |&1:5,r| |
| Hazard Information | Back Directory | [Chemical Properties]
White Crystalline Solid | [Uses]
An antidepressant. A dual serotonin and norepinephrine reuptake inhibitor (SNRI). Used in treatment of stress urinary incontinence. | [Uses]
anti-depressant, analgesic for osteoarthiritis and musculoskeletal pain | [Uses]
Labelled Duloxetine, which is used as an antidepressant. A dual serotonin and norepinephrine reuptake inhibitor (SNRI).
Current lot is 97% d7 with no d0, d1 or d2 | [Uses]
leucotriene antagonist, antiasthmatic | [Uses]
solubility H2O: soluble5 mg/mL (clear solution, warmed) | [Definition]
ChEBI: A duloxetine hydrochloride in which the duloxetine moiety has S configuration. | [Description]
Duloxetine is a selective serotonin (5-HT) and norepinephrine reuptake inhibitor
(SSNRI) for oral administration, and it is currently approved in the US and in
Europe for the treatment of major depressive disorder (MDD) and diabetic peripheral
neuropathic pain (DPN). Additionally, it is indicated for the treatment of
stress urinary incontinence (SUI) in Europe. However, the US approval for SUI is still pending, and Lilly recently withdrew the NDA for this indication. It is expected
that additional clinical trials may be required to secure FDA approval for SUI
treatment due to concern over the potential of duloxetine to prolong the QT interval
in patients taking concomitant CYP2D6 or CYP1A2 inhibitors such as tricyclic
antidepressants, type 1C antiarrhythmics and phenothiazines. Duloxetine is
the second SSNRI to enter the market. Its predecessor, venlafaxine, has been
available since 1994 for the treatment of depression. Duloxetine has a higher affinity
for human norepinephrine (Ki=7.5 nM) and 5-HT transporters (Ki=0.8 nM)
than venlafaxine (Ki=2480nM and 82nM, respectively). Additionally, it shows a
more balanced ratio of 5-HT to norepinephrine uptake inhibition than venlafaxine.
Duloxetine is a moderate inhibitor of dopamine reuptake (Ki=300nM in rat brain
preparations) and shows only weak affinity for muscarinic, α1-and α2 adrenergic
and histamine H1 receptors (Ki=>2.3μ). It is devoid of any activity against
monoamine oxidase. In vivo, duloxetine inhibits 5-HT and norepinephrine uptake in
rats, with ED50 values of 12 mg/kg and 22 mg/kg p.o., respectively, and has no effect
on dopamine uptake at doses up to 30 mg/kg. The antidepressant and central paininhibitory
action of SSNRIs are derived from the inhibition of both 5-HT and
norepinephrine uptake, which increases the availability of neurotransmitters and
ultimately increases serotonergic and noradrenergic function within the CNS. In the
treatment of SUI with duloxetine, the increased neurotransmitter concentration is
believed to increase the tone and contractions of the urethral sphincter at the
opening of the bladder, helping to prevent accidental urine leakage. The absolute
stereochemistry of duloxetine is shown to be S(+). It is prepared in four steps
starting from 2-acetylthiophene. A key intermediate in the synthesis of duloxetine is
(S)-3-dimethylamino-1-(2-thienyl)-1-propanol, which is obtained from the corresponding
ketone, either by reduction with sodium borohydride and subsequent
chiral resolution of the alcohol product with S-mandelic acid, or by enantioselective
reduction with a chiral amine complex of lithium aluminum hydride. Etherification
of this intermediate with 1-fluoronaphthalene, followed by N-demethylation with
trichloroethylchloroformate affords duloxetine. Orally administered duloxetine is
well absorbed, with Cmax achieved in 6 hours post dose. It exhibits dose-proportional
pharmacokinetics at doses of 20–40 mg twice daily, with an average bioavailability
of about 50% and an elimination half-life of about 12 hours. The
volume of distribution is high (1943 L) and steady-state plasma levels are achieved
after 3 days of dosing. Duloxetine is extensively metabolized, mainly by CYP2D6
and CYP1A2. The primary routes of elimination are in the urine (70%) and in the
feces (20%). The recommended dosages of duloxetine are 20 mg to 30 mg twice
daily for treating MDD, 60 mg once daily for treating DPN, and 40 mg twice daily
for treating SUI. In clinical trials with adult MDD outpatients (n =1059), duloxetine
at doses of 40–120 mg daily for 8 to 9 weeks demonstrated superiority over
placebo as measured by improvement in the 17-item Hamilton Depression Rating
Scale total score. In trials involving 791 DPN patients, 60 and 120 mg once daily
dose of duloxetine statistically significantly improved the endpoint mean pain
scores from baseline, and increased the proportion of patients with at least 50%
reduction in pain score from baseline. All doses of duloxetine resulted in superior
results than placebo. In phase III studies of adult women with predominant symptoms of SUI (n=1635), duloxetine 40 mg twice daily reduced the median
incontinence episode frequency from baseline to a significantly greater extent than
placebo (50.0–53.6% versus 27.5–40.0%). In addition, duloxetine recipients experienced
significantly greater increases in their average voiding interval than placebo
recipients (15.0–20.4 versus 1.7–8.5 minutes). The most commonly observed adverse
events associated with duloxetine therapy are nausea, dry mouth, constipation,
decreased appetite, fatigue, somnolence, and increased sweating. As with other
5-HT reuptake inhibitors, duloxetine is contraindicated in patients taking MAO
inhibitors. Additionally, it is contraindicated in patients with uncontrolled narrowangle
glaucoma. | [Originator]
Lilly (US) | [Brand name]
Cymbalta (Lilly). | [General Description]
Pharmaceutical secondary standards for application in quality control provide pharma laboratories and manufacturers with a convenient and cost-effective alternative to the preparation of in-house working standards | [Biochem/physiol Actions]
Duloxetine hydrochloride is a dual serotonin/norepinephrine reuptake inhibitor (SNRI), widely used clinically as an antidepressant and anxiolytic. | [Synthesis]
The
synthesis from Lilly?ˉs group is depicted in Scheme 4.
Friedel-Crafts acylation of thiophene (24) by 3-
chloropropanoyl chloride (25) with SnCl4 as Lewis acid
gave ketone 26 which was then enantioselectively reduced
with (R )-1-methyl-3,3-diphenyl-tetrahydropyrrolo[1,2-
c][1,3,2]oxazaborole (27) in the presence of borane in THF
to give (S)-3-chloro-1-(2-thienyl)-1-propanol (28).
Compound 28 was subjected to Finkelstein reaction to give
(S)-3-iodio-1-(2-thienyl)-1-propanol which was reacted with
methylamine in THF to give compound 29. The alcohol 29
was then used in a nucleophilic displacement reaction with
1-fluoronaphthalene (30) in the presence of sodium hydride
in DMA to give duloxetine free base in 88% yield. Finally,
the free base was treated with HCl to yield duloxetine
hydrochloride (IV).
| [storage]
Room temperature |
| Questions And Answer | Back Directory | [Pharmacological effects]
Duloxetine hydrochloride is the salt form of antidepressant drug duloxetine. It was successfully developed by Eli Lilly Company. Its pharmacological effect is the same as duloxetine. Duloxetine hydrochloride is a new kind of selective dual inhibitor of 5 hydroxy trptamine (5-HT) and norepinephrine reuptake, and thus having antidepressant effect, while also having inhibitory effect on the central pain. Its pharmacological characteristic is being able to inhibit neuronal pre-synaptic membrane’s reuptake on 5-hydroxy trptamine and norepinephrine but instead have a low inhibitory effect on the reuptake of dopamine. Duloxetine hydrochloride is indicated for depression and it is effective in treating both endogenous and non-endogenous depression as well as the feeling of pain associated with depression. It has a therapeutic dose of 60mg/d~120mg/d with a good security and fewer adverse reactions with common adverse reactions such as nausea, dry mouth, constipation, poor appetite, fatigue, sleepiness, and increased sweating. Another indication is the pain caused by diabetic neuropathy.
[Product Name] Cymbalta
[Alias] LY-264453, LY-248686
[Efficacy] antidepressant drug for treatment of stressful urinary incontinence.
[CAS]:136434-34-9
[Development Unit] Japan Shionogi Company, American company Eli-Lilly.
Duloxetine is a selective inhibitor for 5-HT and NE reuptake (SSNRI). The exact mechanism of the anti-depression duloxetine and central analgesic effect is not yet clear. It is thought that it is related to its reinforcing effect on the function of 5 hydroxy trptamine (5-HT) and norepinephrine in the central nervous system. The results of the clinical studies have shown that duloxetine is a strong inhibitor of the neuronal 5-HT and NE reuptake and has a relatively weak inhibitory effect on the reuptake of dopamine. In vitro studies have showed that duloxetine has no significant affinity to dopaminergic receptors, adrenergic receptors, cholinergic receptors, histamine receptors, opiate receptors, glutamate receptors, and the GABA receptors. Duloxetine does not inhibit monoamine oxidase.
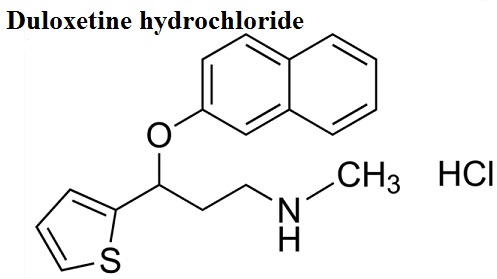
Figure 1: The molecule formula of Duloxetine hydrochloride
| [Pharmacokinetics]
Absorption and Distribution: oral administration of duloxetine hydrochloride enteric-coated capsules can yield a complete absorption with the average lag being two hours before the drug began to be absorbed (TLag). At 6 hours after oral administration, the duloxetine reaches maximum plasma concentration (Cmax) with eating causing no effects on the Cmax, but will delay the peak time to about 6 to 10 hours with slightly lower degree of absorption at about 10%. Compare the evening administration once with morning administration once, the absorption of duloxetine is delayed by 3 hours; apparent elimination increases by one thirds; the apparent volume of distribution is about 1640 L. Duloxetine has a high affinity (> 90%) to the human plasma proteins which mainly binds with albumin and α-acid glycoprotein. There has been no evaluation of whether there exist interactions between duloxetine with other high affinity protein binding drugs. Liver or renal insufficiency does not affect the binding between plasma protein and duloxetine.
Metabolism and Excretion: oral administration of C14-labeled duloxetine can be used to determine their in vivo biotransformation and degradation. The plasma duloxetine only account for about 3% of the total radiolabel, suggesting that duloxetine is extensively metabolized with many kinds of metabolites. The major biotransformation pathway of duloxetine includes naphthyl epoxidization after the binding and further oxidation. Among in vitro tests, CYP2D6 and CYPIA2 can both catalyze the epoxidation of naphthyl with the plasma product including glucuronide-bound 4-hydroxy duloxetine and sulfate-bound 5-hydroxy 6-methoxy duloxetine. It has been separated of various kinds of other metabolites from urine with some appearing only in the small elimination metabolic bypass. Only a small amount (about 1% of the oral dose) of non-metabolized duloxetine prototype is detected in the urine with the majority (about 70% of the oral dose) existing in the form of duloxetine metabolic product which is excreted in urine. About 20% of them is excreted through feces.
The above information is edited by the Chemicalbook of Dai Xiongfeng.
| [Indications]
It is used for the treatment of depression and the treatment of stress urinary incontinence.
In 2004, Japan Shionogi Pharmaceutical Co., Ltd. had obtained the authorization of Eli Lilly Company of US to perform further development on the duloxetine which has dual inhibitory effect on the reuptake of both 5 hydroxy trptamine (5-HT) and norepinephrine (NE). Existing studies have shown that this drug has certain efficacy in treating tension urinary incontinence, depression, and obesity. Currently, clinical trials of the drug for treatment of tension urinary incontinence and depression have all been completed.
| [Precautions]
1. Liver and kidney dysfunction can significantly reduce the metabolism and clearance of duloxetine. Thus it is not recommended for such patients to apply duloxetine.
2. The interaction between duloxetine and alcohol may cause liver damage. Thus it is not generally recommended for the treatment of patients with alcoholism.
3. In the clinical trials, when compared with placebo, duloxetine causes the mean systolic blood pressure increase by 2mmHg, and the average diastolic blood pressure increase by 0.5mmHg. We recommend regular measurement of blood pressure both before and during treatment and therapy.
| [Drug Interactions]
Duloxetine is metabolized through CYP2D6 and CYP1A2 metabolism with moderate inhibition of CYP2D6 but no inhibition and induction effects on CYP1A2 and CYP3A4. We should be cautious when apply it together with other drugs which are also metabolized through CYP2D6 and of narrow therapeutic window (such as: TCAs, Ic antiarrhythmic drugs, and phenothiazines).
| [Medication of Special Populations]
[Medication of Pregnant women and lactating women] It is not clear whether it is safe when applied this drug for pregnant women of pregnancy category. Therefore, for pregnant women, they should weigh both the advantages and disadvantages to decide whether they should administrate this drug. Only with potential benefits higher than risks can they apply it. Otherwise, they should not administrate this drug during pregnancy and lactation period.
[Pediatric administration] There has been no enough clinical experience on the application on children.
[Elderly people] Clinical studies have not observed a significant difference of the security and efficacy between the elderly population and young population. But it can’t be exclude that the sensitivity of some elderly patients can be increased.
| [Contraindications]
1. It should be contraindicated in patients known to be allergic to duloxetine or any of the inactive ingredients contained in the product.
2. It should be prohibited to apply it in combination with monoamine oxidase inhibitors (MAOIs). It is also not allowed to administrate this drug within 14 days after the withdrawal of MAOIs; According to the half-life of duloxetine, only after the five days of the withdrawal of duloxetine can the patients begin to administrate MAOIs.
3. Clinical trials have shown that duloxetine can increase the risk of mydriasis and therefore the angle-closure glaucoma patients without control should avoid applying duloxetine.
|
|
|