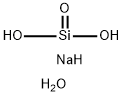| Identification | More | [Name]
SODIUM METASILICATE NONAHYDRATE | [CAS]
13517-24-3 | [Synonyms]
DISODIUM TRIOXOSILICATE, NONAHYDRATE
SODIUM META-SILICATE, 9-HYDRATE
SODIUM METASILICATE NONAHYDRATE
SODIUM M-SILICATE, HYDROUS
SODIUM SILICATE
SODIUM SILICATE, META NONAHYDRATE
Silicicacid(H2SiO3)disodiumsaltnonahydrate
Silicicacid,disodiumsalt,nonahydrate
Sodiummetasilicate(2-)nonahydrate
Sodiumsilicate,nonahydrate
Sodium metasilicate, nonahydrate, 43.5-45.0% total solids
SODIUM METASILICATE NONAHYDRATE PLANT*CE LL CULTURE
Sodiummetasilicateenneahydrate
Sodium metasilicate nonahydrate, 44-47.5% total solids
SODIUMMETA-SILICATE,NONAHYDRATE,CRYSTAL,REAGENT
SILICICACID,DISODIUMSALT(CRYSTALLINENONAHYDRATE) | [EINECS(EC#)]
229-912-9 | [Molecular Formula]
H18Na2O12Si | [MDL Number]
MFCD00149175 | [Molecular Weight]
284.2 | [MOL File]
13517-24-3.mol |
| Hazard Information | Back Directory | [Chemical Properties]
white crystals | [Definition]
ChEBI: A hydrate that is the nonahydrate form of sodium silicate. | [Uses]
Sodium Metasilicate Nonahydrate is used in preparation method and application of Cationic Polyacrylamide modified Silicon Dioxide/Calcium Inosilicate material in wastewater treatment. |
| Safety Data | Back Directory | [Hazard Codes ]
C | [Risk Statements ]
R34:Causes burns.
R37:Irritating to the respiratory system. | [Safety Statements ]
S13:Keep away from food, drink and animal feeding stuffs .
S36/37/39:Wear suitable protective clothing, gloves and eye/face protection .
S45:In case of accident or if you feel unwell, seek medical advice immediately (show label where possible) . | [RIDADR ]
UN 3253 8/PG 3
| [WGK Germany ]
1
| [HazardClass ]
8 | [PackingGroup ]
III | [HS Code ]
28391100 |
| Questions And Answer | Back Directory | [Description]
Sodium metasilicate non-hydrate is the non-hydrate form of sodium metasilicate. Sodium metasilicate (chemical formula: Na2SiO3) is a kind of sodium silicate, appearing as a powdered or flaked solid. It is used in cements, passive fire protection, textile and lumber processing, refractories, and automobiles. It has various kinds of applications. The major applications are in detergents, paper, water treatment, and construction materials as well as dye auxiliary. In addition, it also has many niche and hobby applications such as used in food preservation, metal repair, automotive repair, home-brewing, aquaculture and pottery. It is manufactured through the reaction between sodium carbonate and silicon dioxide.
| [References]
https://en.wikipedia.org/wiki/Sodium_silicate
https://pubchem.ncbi.nlm.nih.gov/compound/Sodium_metasilicate
|
|
|