| Identification | More | [Name]
TUNGSTEN(VI) CHLORIDE | [CAS]
13283-01-7 | [Synonyms]
TUNGSTEN(+6)CHLORIDE
TUNGSTEN CHLORIDE
TUNGSTEN(VI) CHLORIDE
(oc-6-11)-tungstenchlorid
(oc-6-11)-tungstenchloride(wcl6
Hexachlorotungsten
Tungsten chloride (WCl6), (OC-6-11)-
Tungsten chloride, (oc-6-11)-
Tungsten chloride, alpha
WCl6
Wolfram hexachloride
wolframhexachloride
Tungsten(VI) chloride, 99.9+% metals basis
TUNGSTEN (VI) CHLORIDE 99.5+%
TUNGSTEN (VI) CHLORIDE 99%
Tungsten(VI) chloride, 20 mesh, 99%
Tungsten(VI) chloride, 99.9+%
Tungsten (VI) chloride (Tungsten hexachloride)
Tungsten(VI)chloride(99.9%-W)
(oc-6-11)-tungsten chloride | [EINECS(EC#)]
236-293-9 | [Molecular Formula]
Cl6W | [MDL Number]
MFCD00011463 | [Molecular Weight]
396.56 | [MOL File]
13283-01-7.mol |
| Chemical Properties | Back Directory | [Appearance]
grey to dark grey-blue powder | [Melting point ]
275 °C(lit.)
| [Boiling point ]
347 °C(lit.)
| [density ]
3.52 g/mL at 25 °C(lit.)
| [vapor pressure ]
43 mm Hg ( 215 °C)
| [Fp ]
346°C | [solubility ]
Soluble in carbon disulfide, carbon tetrachloride and phosphorus oxychloride. | [form ]
powder
| [color ]
Gray to dark gray-blue | [Water Solubility ]
soluble organic solvents such as ethanol [HAW93] | [Sensitive ]
Moisture Sensitive | [Exposure limits]
ACGIH: TWA 3 mg/m3
NIOSH: TWA 5 mg/m3; TWA 1 mg/m3; STEL 10 mg/m3; STEL 3 mg/m3 | [InChI]
InChI=1S/6ClH.W/h6*1H;/q;;;;;;+6/p-6 | [InChIKey]
KPGXUAIFQMJJFB-UHFFFAOYSA-H | [SMILES]
[W](Cl)(Cl)(Cl)(Cl)(Cl)Cl | [CAS DataBase Reference]
13283-01-7(CAS DataBase Reference) | [EPA Substance Registry System]
Tungsten chloride (WCl6), (OC-6-11)- (13283-01-7) |
| Hazard Information | Back Directory | [Chemical Properties]
dark grey-violet crystalline powder | [Uses]
A precursor to tungsten based carbenes. | [Uses]
Precursor to tungsten-based carbenes which catalyze the olefin metathesis reactions of 1- and 2-octene. | [Description]
Tungsten(VI) chloride, WCl6, is a dark violet blue species that exists as a volatile solid under standard conditions. It
is an important starting reagent in the preparation of tungsten compounds. As a d0 ion, W(VI) forms diamagnetic derivatives. WCl6 is octahedral with equivalent W-Cl distances of 2.24-2.26 ? . | [Definition]
ChEBI: Tungsten hexachloride is a tungsten coordination entity. | [Preparation]
WCl6 can be prepared by chlorinating
tungsten metal in a sealed tube at 600℃.
W +3Cl2→WCl6 | [Reactions]
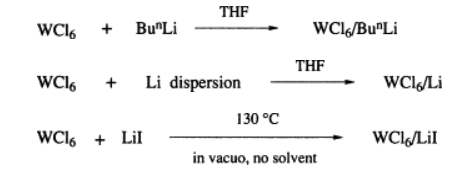
The tungsten reagents were prepared from tungsten hexachloride (Tungsten(VI) Chloride) in three ways: (i) with butyllithium; (ii) with a lithium dispersion; and (iii) with lithium iodide. In each case the ratio of WCl6 to lithium reagent is important.
| [Purification Methods]
Sublime it in a stream of Cl2 in a high temperature furnace and collect it in a receiver cooled in a Dry Ice-acetone bath in an inert atmosphere because it is sensitive to moisture. It is soluble in CS2, CCl4, CHCl3, POCl3, *C6H6, pet ether and Me2CO. Its solutions decompose on standing. Good crystals can be obtained by heating WCl6 in CCl4 to 100o in a sealed tube, followed by slow cooling (tablets of four-sided prisms). Store it in a desiccator over H2SO4 in the dark. [Leitzke & Holt Inorg Synth III 163 1950, Parterfield & Tyree Inorg Synth IX 133 1967, Hein & Herzog in Handbook of Preparative Inorganic Chemistry (Ed. Brauer) Academic Press Vol II p 1420 1965.] | [Flammability and Explosibility]
Notclassified(100%) | [reaction suitability]
reagent type: catalyst
core: tungsten |
| Safety Data | Back Directory | [Hazard Codes ]
C | [Risk Statements ]
R34:Causes burns. | [Safety Statements ]
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice .
S36/37/39:Wear suitable protective clothing, gloves and eye/face protection .
S45:In case of accident or if you feel unwell, seek medical advice immediately (show label where possible) . | [RIDADR ]
UN 3260 8/PG 2
| [WGK Germany ]
3
| [RTECS ]
YO7710000
| [F ]
1-10-16 | [TSCA ]
Yes | [HazardClass ]
8 | [PackingGroup ]
II | [HS Code ]
28273990 |
|
|