| Identification | More | [Name]
Bis(cyclopentadienyl)zirconium dichloride | [CAS]
1291-32-3 | [Synonyms]
BIS(CYCLOPENTADIENYL)ZIRCONIUM DICHLORIDE
BIS(CYCLOPENTADIENYL)ZIRCONIUMICHLORIDE
BIS(CYCLOPENTADIENYL)ZIRCONIUM(IV) DICHLORIDE
DICHLORODICYCLOPENTADIENYLZIRCONIUM
DICYCLOPENTADIENYLZIRCONIUM DICHLORIDE
ZIRCONOCENE DICHLORIDE
bis(η-cyclopentadienyl)zirconiumchloride
bis(η-cyclopentadienyl)-zirconiumchloride
Bis-pi-cyclopentadienyldichlorozirconium
Dichlorobis(cyclopentadienyl) zirconium
dichlorobis(eta5-2,4-cyclopentadien-1-yl)-zirconiu
dichlorobis(η5-2,4-cyclopentadien-1-yl)-Zirconium
dichloro-di-pi-cyclopentadienyl-zirconiu
Dichlorodi-pi-cyclopentadienylzirconium
Dichlorodi-pi-dicyclopentadienylzirconium
Dichlorozirconocene
Dicyclopentadienyldichlorozirconium
Zirconcene dichloride
Zirconium dicyclopentadiene dichloride
Zirconium, dichlorobis(2,4-cyclopentadien-1-yl)- | [EINECS(EC#)]
215-066-8 | [Molecular Formula]
C10H10Cl2Zr | [MDL Number]
MFCD00003726 | [Molecular Weight]
292.32 | [MOL File]
1291-32-3.mol |
| Chemical Properties | Back Directory | [Appearance]
White crystals. Soluble in
polar organic solvents. Stable in dry air, very slowly
hydrolyzes in moist air. | [Melting point ]
242-245 °C(lit.)
| [Boiling point ]
124-125°C/15mm | [Fp ]
124-125°C/15mm | [storage temp. ]
Refrigerator (+4°C) | [form ]
Powder | [color ]
white to off-white | [Water Solubility ]
hydrolysis | [Hydrolytic Sensitivity]
4: no reaction with water under neutral conditions | [Sensitive ]
Air & Moisture Sensitive | [Exposure limits]
ACGIH: TWA 5 mg/m3; STEL 10 mg/m3
NIOSH: IDLH 25 mg/m3; TWA 5 mg/m3; STEL 10 mg/m3 | [Uses]
Rubber accelerator, component of a catalyst
system for polymerization of vinyl monomers, curing
agent for water-repellent silicone materials,
agent for plating with zirconium. | [CAS DataBase Reference]
1291-32-3(CAS DataBase Reference) | [NIST Chemistry Reference]
Bis(cyclopentadienyl)zirconium dichloride(1291-32-3) | [EPA Substance Registry System]
1291-32-3(EPA Substance) |
| Safety Data | Back Directory | [Hazard Codes ]
Xi | [Risk Statements ]
R36/37/38:Irritating to eyes, respiratory system and skin . | [Safety Statements ]
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice .
S36:Wear suitable protective clothing . | [RIDADR ]
UN3261 | [WGK Germany ]
3
| [RTECS ]
ZH7525000
| [F ]
8-10-21 | [TSCA ]
Yes | [HazardClass ]
8 | [PackingGroup ]
II | [HS Code ]
29319090 | [Safety Profile]
Poison by
intraperitoneal route. Mutation data
reported. When heated to decomposition it
emits toxic fumes of Zr and Cl-. |
| Hazard Information | Back Directory | [General Description]
White crystals or off-white crystalline solid. | [Reactivity Profile]
ZIRCONOCENE DICHLORIDE(1291-32-3) is incompatible with water, acids, bases, alcohols and halogens. | [Air & Water Reactions]
This compound is extremely unstable when exposed to air. Decomposes in water . | [Fire Hazard]
Flash point data for this material are not available, but ZIRCONOCENE DICHLORIDE is probably combustible. | [Description]
Bis(cyclopentadienyl)zirconium(IV) Dichloride or zirconocene dichloride is one of numerous organo-metallic compounds (also known as metalorganic, organo-inorganic and metallo-organic compounds) sold by American Elements under the trade name AE Organo-MetallicsTM. As is the case for other transition metal metallocenes, bis(cyclopentadienyl)zirconium(IV) dichloride is most often used as a catalyst. | [Chemical Properties]
White crystals. Soluble in
polar organic solvents. Stable in dry air, very slowly
hydrolyzes in moist air. | [Application]
Used to promote greener amidations of carboxylic acids and amines in catalytic amounts. This technology avoids the requirement of preactivation of the carboxylic acid or use of coupling reagents.
Direct amide formation from unactivated carboxylic acids and amines.
Useful in the synthesis of a wide range of early-transition-metal complexes and organometallic compounds. | [Preparation]
Synthesis of zirconium dichlorodichloride
A stirring magnet was placed in a 500 mL three-necked flask, fitted with a constant pressure dropping funnel and reflux condenser, evacuated, and replaced with pure nitrogen three times. The flask was added with 50mL of toluene and 22.3g (0.1mol) of ZrCl4, and the suspension was made by turning on the stirring. Add the THF solution of sodium cyclopentadienylide to a constant pressure dropping funnel, add dropwise at room temperature, keep the reaction solution slightly boiling, after the dropwise addition, continue the reaction for 2h. Under the heating of oil bath at 50℃, evaporate the solvent under reduced pressure to obtain a yellow solid. Put the solid into a soxhlet extractor and extract with CHCl3. Most of the solvent in the extract was evaporated under reduced pressure, and the solid was precipitated after cooling and filtered. The product was washed with a small amount of CHCl3 and dried under vacuum, yielding 20.2g of white crystals, 69% yield. | [Reactions]
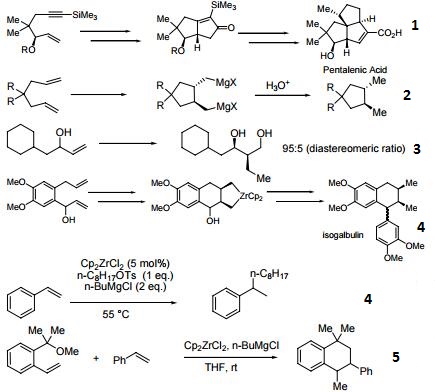
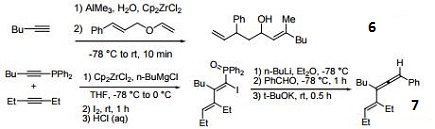
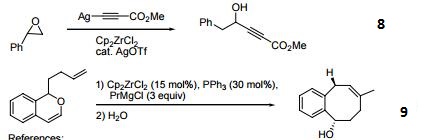
Reagent for the conversion of enynes to bicyclic cyclopentenones.
Precursor for the cyclization of dienes to cyclopentane and cyclohexane derivatives.
Precatalyst for the alkylation of olefins.
Precursor to zirconocene complexes of unsaturated organic molecules.
Catalyst for the coupling of alkoxymethyl-substituted styrene derivatives.
Reagent for the carboalumination-Claisen rearrangement-carbonyl addition cascade reaction.
Useful for the preparation of vinyl allenes.
Reagent for the alkynylation of epoxides.
Catalyst for the formation of carbocycles from cyclic enol ether.
 | [Hazard]
Toxic by inhalation and skin contact, irritant
to eyes and mucous membranes. | [Purification Methods]
Recrystallise the dichloride from CHCl3 or xylene and dry it in a vacuum. 1H NMR (CDCl3) : 6.52 from Me4Si. Store it dry in the dark under N2. [Reid et al. Aust J Chem 18 173 1965, Beilstein 16 IV 1770.] |
|
|