| Identification | More | [Name]
Testosterone phenylpropionate | [CAS]
1255-49-8 | [Synonyms]
17b-hydroxyandrost-4-en-3-one 3-phenylpropionate
3-PHENYL-PROPIONIC ACID (8R,9S,10R,13S,14S,17S)-10,13-DIMETHYL-3-OXO-2,3,6,7,8,9,10,11,12,13,14,15,16,17-TETRADECAHYDRO-1H-CYCLOPENTA[A]PHENANTHREN-17-YL ESTER
4-ANDROSTEN-17BETA-OL-3-ONE 17-PHENYLPROPIONATE
4-ANDROSTEN-17BETA-OL-3-ONE 3-PHENYLPROPIONATE
4-ANDROSTEN-17-BETA-OL-3-ONE PHENYLPROPIONATE
TESTOSTERONE 17-PHENYLPROPIONATE
TESTOSTERONE-3-PHENYLPROPIONATE
TESTOSTERONE HYDROCINNAMATE
TESTOSTERONE PHENPROPIONATE
TESTOSTERONE PHENYLPROPIONATE
retandrol
testosterone17-phenylpropionate--dea*scheduleii
17beta-hydroxyandrost-4-en-3-one 3-phenylpropionate
TESTOSTERONE 17-PHENYLPROPIONATE--DEA*SC HEDULE III
Stosterone
Androst-4-en-3-one, 17-(1-oxo-3-phenylpropoxy)-, (17.beta.)-
4-androsten-17β-ol-3-one 17-phenylpropionate
Testosterone 17B-phenylpropionate
4-Androstene-17-Oxopropoxyl-3-one-17-phenylpropionate | [EINECS(EC#)]
215-014-4 | [Molecular Formula]
C28H36O3 | [MDL Number]
MFCD00056484 | [Molecular Weight]
420.58 | [MOL File]
1255-49-8.mol |
| Chemical Properties | Back Directory | [Melting point ]
116.5 °C | [Boiling point ]
497.74°C (rough estimate) | [density ]
1.0043 (rough estimate) | [refractive index ]
1.5460 (estimate) | [solubility ]
DMF: 25 mg/mL; DMSO: 5 mg/mL; Ethanol: 15 mg/mL; Ethanol:PBS (pH 7.2)(1:2): 0.3 mg/mL | [form ]
neat | [Water Solubility ]
2.25mg/L(25 ºC) | [InChIKey]
HHSXYDOROIURIP-FEZCWRLCSA-N | [SMILES]
[C@@]12(CC[C@]3(C)[C@H](CC[C@@]3([H])[C@@]1(CCC1=CC(=O)CC[C@]21C)[H])OC(=O)CCC1C=CC=CC=1)[H] |&1:0,3,5,8,10,19,r| | [CAS DataBase Reference]
1255-49-8(CAS DataBase Reference) |
| Safety Data | Back Directory | [Hazard Codes ]
Xn | [Risk Statements ]
R20/21/22:Harmful by inhalation, in contact with skin and if swallowed .
R40:Limited evidence of a carcinogenic effect. | [Safety Statements ]
S22:Do not breathe dust .
S36:Wear suitable protective clothing . | [WGK Germany ]
3
| [RTECS ]
XA3105000
|
| Hazard Information | Back Directory | [Description]
Testosterone phenylpropionate (BAN; TPP) (brand name Testolent), or testosterone phenpropionate, also known as testosterone hydrocinnamate, is a synthetic anabolic-androgenic steroid (AAS) and an androgen ester – specifically, the C17β phenylpropionate ester of testosterone – which was formerly marketed in Romania. It was first synthesized in 1951 and was first described in the literature by 1953. The medication was an ingredient of several isolated AAS commercial products, but was never widely used. Testosterone phenylpropionate was also notably a component of Sustanon and Omnadren, as well as of Estandron Prolongatum, Lynandron Prolongatum, and Mixogen. TPP was previously available in Great Britain. | [Uses]
Testosterone 17-Phenylpropionate, is one of the 17b-fatty acid esters of testosterone, that is increasingly misused by athletes in an attempt to improve sports performance, and thus can be used in doping control. It can also be used as a therapy in glucocorticoid-treated patients to increase the circulating testosterone concentration. | [Definition]
ChEBI: Testosterone phenylpropionate is a steroid ester. | [Biological Functions]
Testosterone phenylpropionate is a synthetic anabolic-androgenic steroid (AAS) and an androgen ester. It was first reported in the scientific literature in 1955 and was an ingredient of several isolated AAS commercial products, but was never widely used. Testosterone phenylpropionate was also notably a component of Sustanon and Omnadren. | [Synthesis]
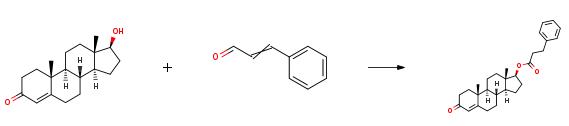 In a 100 ml reaction flask, 0.397 g (3 mmol) of cinnamaldehyde was added.0.411g (1.5mmol) Nandrolone, 0.06g (0.3mmol) [Bmim][OAc] catalyst, 10ml 1,4-dioxane, heated to 120 ?? C and then reacted for 2h, after the reaction was completed, cooled to room temperature, added 30 ml of water and 20 ml of ethyl acetate were extracted, and the phases were allowed to stand. The organic phase was concentrated and crystallized to obtain the target product. The yield was 64%. In a 100 ml reaction flask, 0.397 g (3 mmol) of cinnamaldehyde was added.0.411g (1.5mmol) Nandrolone, 0.06g (0.3mmol) [Bmim][OAc] catalyst, 10ml 1,4-dioxane, heated to 120 ?? C and then reacted for 2h, after the reaction was completed, cooled to room temperature, added 30 ml of water and 20 ml of ethyl acetate were extracted, and the phases were allowed to stand. The organic phase was concentrated and crystallized to obtain the target product. The yield was 64%. |
|
|